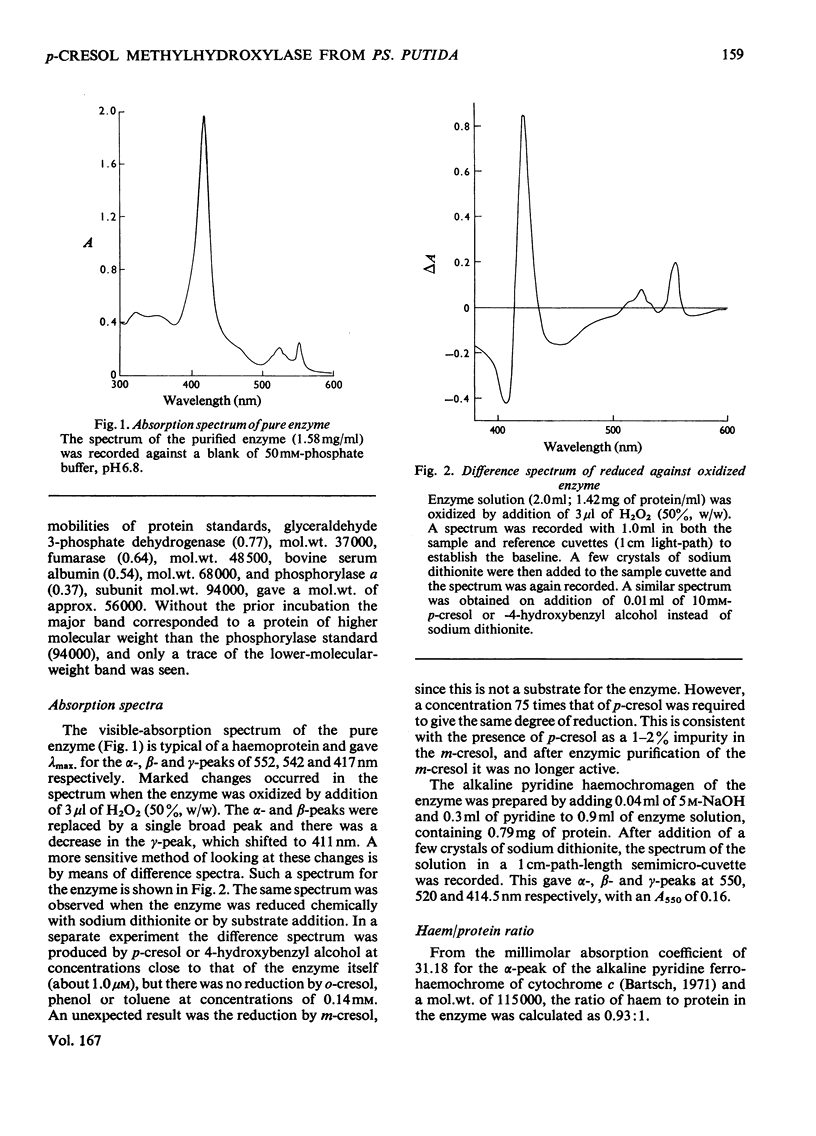
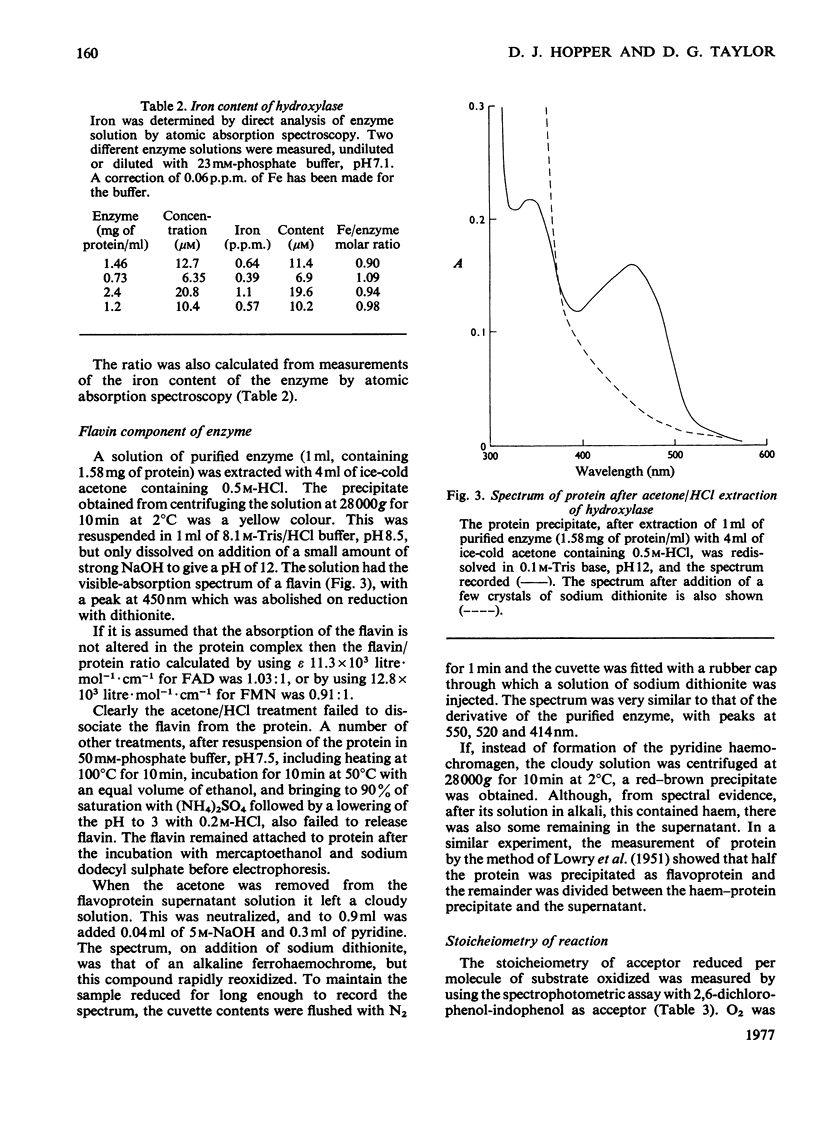
Abstract
The enzyme that catalyses the hydroxylation of the methyl group of p-cresol was purified from Pseudomonas putida. It has mol.wt. 115000 and appears to contain two subunits of equal molecular weight. One subunit is a c-type cytochrome and the other is a flavoprotein. Reduction of the cytochrome occurred on addition of substrate. The same enzyme catalyses both p-cresol hydroxylation and the further oxidation of the product, 4-hydroxybenzyl alcohol. The stoicheiometry of acceptor reduced per molecule of substrate oxidized is that for two dehydrogenation reactions. The Km for p-cresol is 7.3 x 10(-6) M and that for 4-hydroxybenzyl alcohol is 47.6 x 10(-6) M. The enzyme, which is assayed with phenazine methosulphate as electron acceptor, was stimulated by particulate material, which probably contains the acceptor in vivo.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG J. M. THE MOLAR EXTINCTION COEFFICIENT OF 2,6-DICHLOROPHENOL INDOPHENOL. Biochim Biophys Acta. 1964 Apr 4;86:194–197. doi: 10.1016/0304-4165(64)90180-1. [DOI] [PubMed] [Google Scholar]
- Bernhardt F. H., Pachowsky H., Staudinger H. A 4-methoxybenzoate O-demethylase from Pseudomonas putida. A new type of monooxygenase system. Eur J Biochem. 1975 Sep 1;57(1):241–256. doi: 10.1111/j.1432-1033.1975.tb02296.x. [DOI] [PubMed] [Google Scholar]
- Chapman P. J., Hopper D. J. The bacterial metabolism of 2,4-xylenol. Biochem J. 1968 Dec;110(3):491–498. doi: 10.1042/bj1100491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAGLEY S., PATEL M. D. Oxidation of p-cresol and related compounds by a Pseudomonas. Biochem J. 1957 Jun;66(2):227–233. doi: 10.1042/bj0660227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- GHOLSON R. K., BAPTIST J. N., COON M. J. HYDROCARBON OXIDATION BY A BACTERIAL ENZYME SYSTEM. II. COFACTOR REQUIREMENTS FOR OCTANOL FORMATION FROM OCTANE. Biochemistry. 1963 Sep-Oct;2:1155–1159. doi: 10.1021/bi00905a043. [DOI] [PubMed] [Google Scholar]
- Hopper D. J., Chapman P. J. Gentisic acid and its 3- and 4-methyl-substituted homologoues as intermediates in the bacterial degradation of m-cresol, 3,5-xylenol and 2,5-xylenol. Biochem J. 1971 Mar;122(1):19–28. doi: 10.1042/bj1220019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper D. J. The hydroxylation of P-cresol and its conversion to P-hydroxybenzaldehyde in Pseudomonas putida. Biochem Biophys Res Commun. 1976 Mar 22;69(2):462–468. doi: 10.1016/0006-291x(76)90544-1. [DOI] [PubMed] [Google Scholar]
- Jones M. V. Cytochrome c linked nicotinic acid hydroxylase in Pseudomonas ovalis Chester. FEBS Lett. 1973 Jun 1;32(2):321–324. doi: 10.1016/0014-5793(73)80864-6. [DOI] [PubMed] [Google Scholar]
- Jones M. V., Hughes D. E. The oxidation of nicotinic acid by Pseudomonas ovalis Chester. The terminal oxidase. Biochem J. 1972 Sep;129(3):755–761. doi: 10.1042/bj1290755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- ROGERS L. J. A SIMPLE APPARATUS FOR DISC ELECTROPHORESIS. Biochim Biophys Acta. 1965 Mar 29;94:324–329. doi: 10.1016/0926-6585(65)90041-5. [DOI] [PubMed] [Google Scholar]
- ROSENBERGER R. F., ELSDEN S. R. The yields of Streptococcus faecalis grown in continuous culture. J Gen Microbiol. 1960 Jun;22:726–739. doi: 10.1099/00221287-22-3-726. [DOI] [PubMed] [Google Scholar]
- Ribbons D. W. Stoicheiometry of O-demethylase activity in Pseudomonas aeruginosa. FEBS Lett. 1970 May 25;8(2):101–104. doi: 10.1016/0014-5793(70)80235-6. [DOI] [PubMed] [Google Scholar]
- Rogoziński J. Molecular properties of the inducible lupanine hydroxylase from growing cultures of Pseudomonas lupanini. Acta Biochim Pol. 1975;22(1):57–66. [PubMed] [Google Scholar]
- Walker W. H., Kenney W. C., Edmondson D. E., Singer T. P., Cronin J. R., Hendriks R. The covalently bound flavin of Chromatium cytochrome c552. 1. Evidence for cysteine thiohemiacetal at the 8 alpha position. Eur J Biochem. 1974 Oct 2;48(2):439–448. doi: 10.1111/j.1432-1033.1974.tb03784.x. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]
- Yamanaka T. The subunits of Chlorobium flavocytochrome c. J Biochem. 1976 Mar;79(3):655–660. doi: 10.1093/oxfordjournals.jbchem.a131110. [DOI] [PubMed] [Google Scholar]


