Abstract
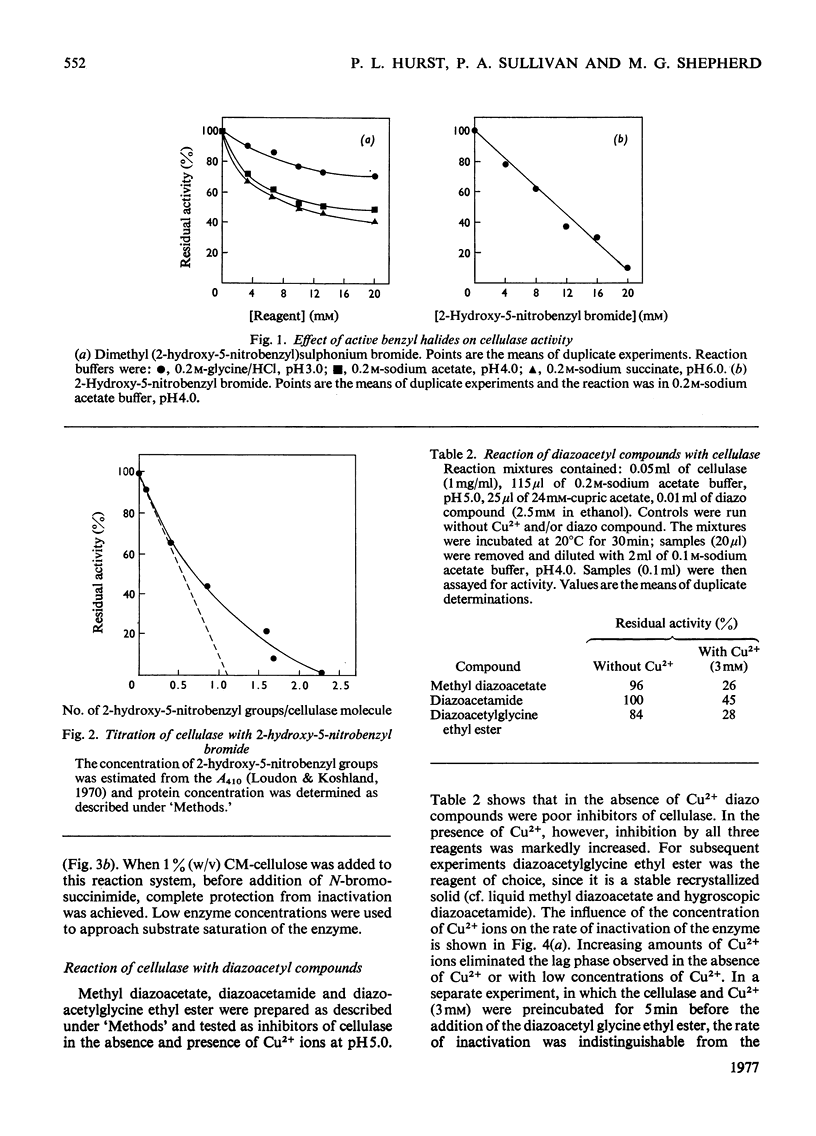
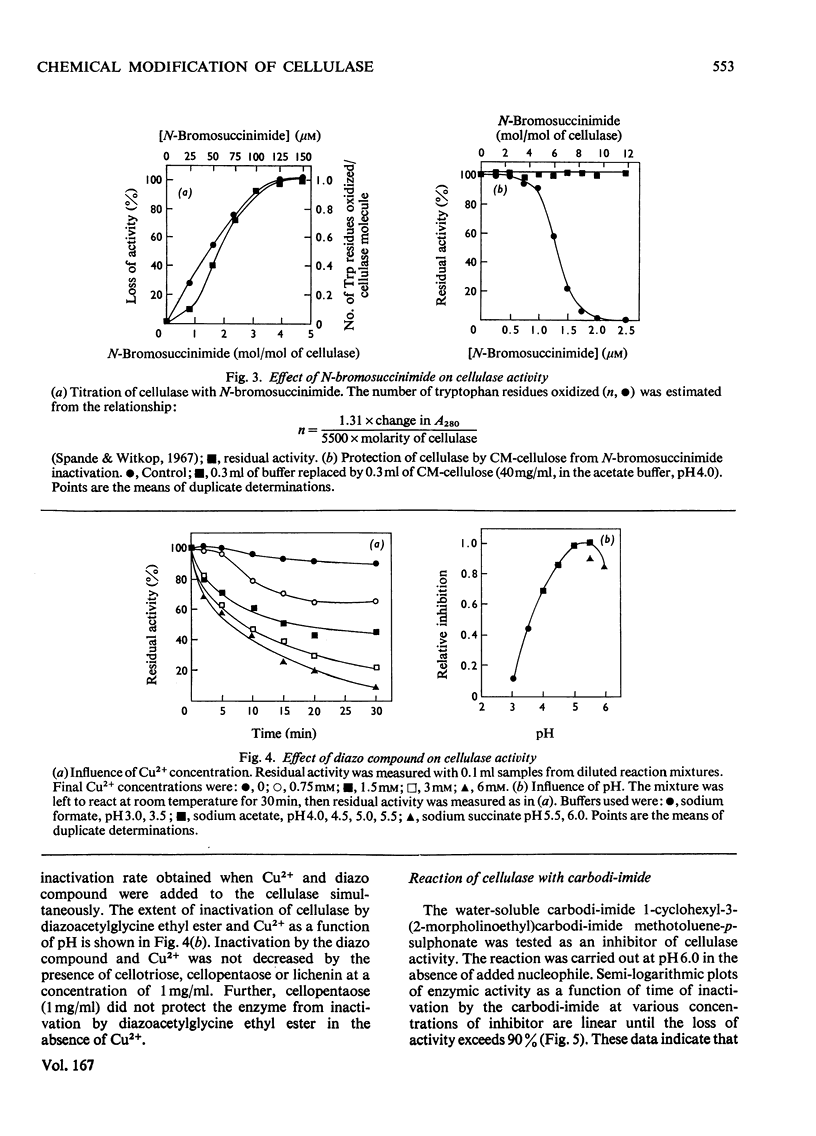
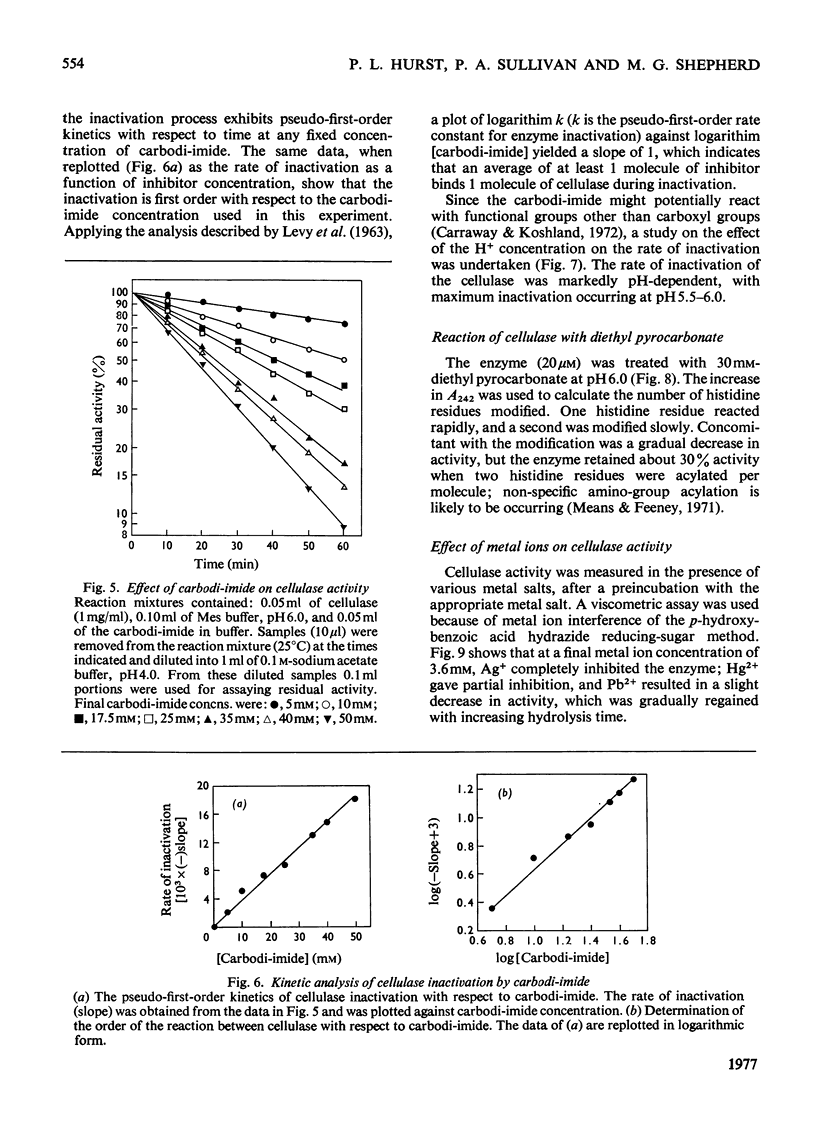
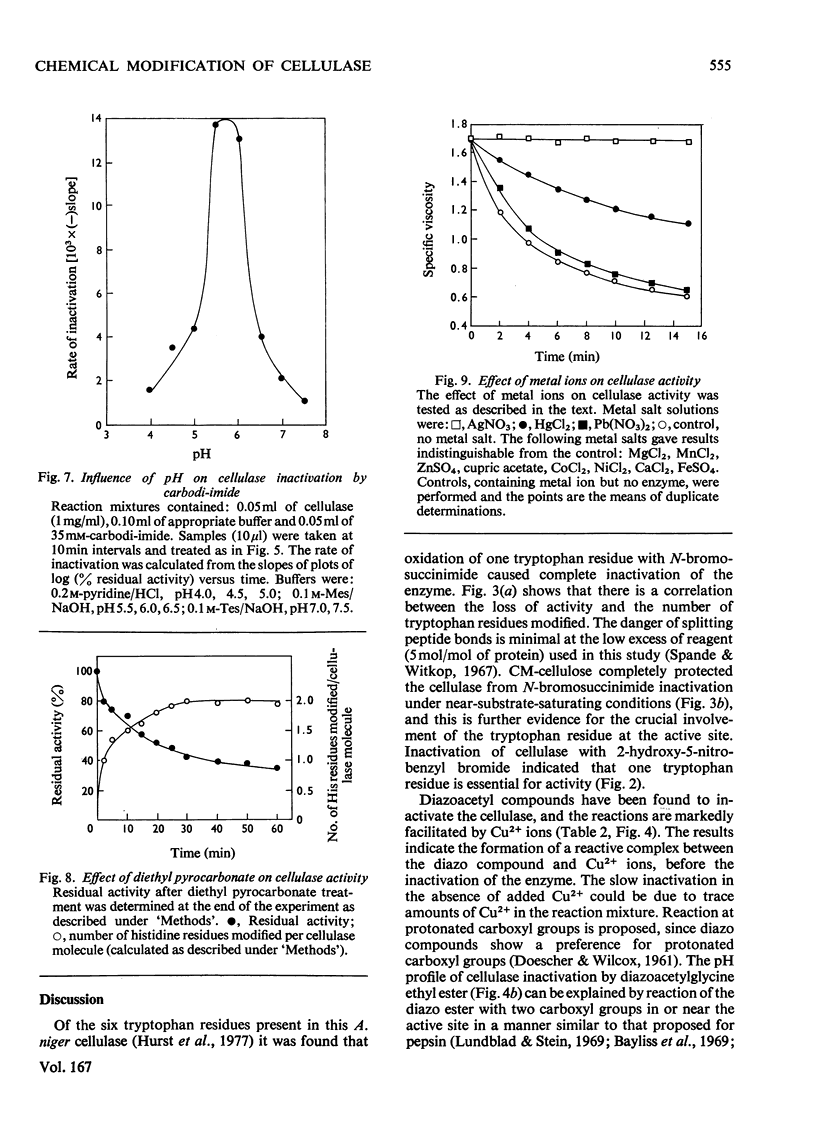
N-Bromosuccinimide completely inactivated the cellulase, and titration experiments showed that oxidation of one tryptophan residue per cellulase molecule coincided with 100% inactivation. CM-cellulose protected the enzyme from inactivation by N-bromosuccinimide. The cellulase was inhibited by active benzyl halides, and reaction with 2-hydroxy-5-nitrobenzyl bromide resulted in the incorporation of 2.3 hydroxy-5-nitrobenzyl groups per enzyme molecule; one tryptophan residue was shown to be essential for activity. Diazocarbonyl compounds in the presence of Cu2+ ions inhibited the enzyme. The pH-dependence of inactivation was consistent with the reaction occurring with a protonated carboxyl group. Carbodi-imide inhibited the cellulase, and kinetic analysis indicated that there was an average of 1 mol of carbodi-imide binding to the cellulase during inactivation. Treatment of the cellulase with diethyl pyrocarbonate resulted in the modification of two out of the four histidine residues present in the cellulase. The modified enzyme retained 40% of its original activity. Inhibition of cellulase activity by the metal ions Ag+ and Hg2+ was ascribed to interaction with tryptophan residues, rather than with thiol groups.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayliss R. S., Knowles J. R., Wybrandt G. B. An aspartic acid residue at the active site of pepsin. The isolation and sequence of the heptapeptide. Biochem J. 1969 Jun;113(2):377–386. doi: 10.1042/bj1130377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOSCHER M. S., WILCOX P. E. Chemical derivatives of alpha-chymotrypsinogen IV. A comparison of the reactions of alpha-chymotrypsinogen and of simple carboxylic acids with diazoacetamide. J Biol Chem. 1961 May;236:1328–1337. [PubMed] [Google Scholar]
- Eriksson K. E., Pettersson G. Studies on cellulolytic enzymes. V. Some structural properties of the cellulase from Penicillium notatum. Arch Biochem Biophys. 1968 Mar 20;124(1):160–166. doi: 10.1016/0003-9861(68)90316-0. [DOI] [PubMed] [Google Scholar]
- Halliwell G., Riaz M. Interactions between components of the cellulase complex of Trichoderma koningii on native substrates. Arch Mikrobiol. 1971;78(4):295–309. doi: 10.1007/BF00412270. [DOI] [PubMed] [Google Scholar]
- Hoare D. G., Koshland D. E., Jr A method for the quantitative modification and estimation of carboxylic acid groups in proteins. J Biol Chem. 1967 May 25;242(10):2447–2453. [PubMed] [Google Scholar]
- Horton H. R., Tucker W. P. Dimethyl(2-hydroxy-5-nitrobenzyl)sulfonium salts. Water-soluble environmentally sensitive protein reagents. J Biol Chem. 1970 Jul 10;245(13):3397–3401. [PubMed] [Google Scholar]
- Hurst P. L., Nielsen J., Sullivan P. A., Shepherd M. G. Purification and properties of a cellulase from Aspergillus niger. Biochem J. 1977 Jul 1;165(1):33–41. doi: 10.1042/bj1650033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Legler G., Bause E. Epoxyalkyl oligo-(1 leads to 4)- -D-glucosides as active-site-directed inhibitors of cellulases. Carbohydr Res. 1973 May;28(1):45–52. doi: 10.1016/s0008-6215(00)82855-4. [DOI] [PubMed] [Google Scholar]
- Loudon G. M., Koshland D. E., Jr The chemistry of a reporter group: 2-hydroxy-5-nitrobenzyl bromide. J Biol Chem. 1970 May 10;245(9):2247–2254. [PubMed] [Google Scholar]
- Lundblad R. L., Stein W. H. On the reaction of diazoacetyl compounds with pepsin. J Biol Chem. 1969 Jan 10;244(1):154–160. [PubMed] [Google Scholar]
- Pettersson G. Structure and function of a cellulase from Penicillium notatum as studies by chemical modification and solvent accessibility. Arch Biochem Biophys. 1968 Sep 10;126(3):776–784. doi: 10.1016/0003-9861(68)90471-2. [DOI] [PubMed] [Google Scholar]
- RIEHM J. P., SCHERAGA H. A. STRUCTURAL STUDIES OF RIBONUCLEASE. XVII. A REACTIVE CARBOXYL GROUP IN RIBONUCLEASE. Biochemistry. 1965 Apr;4:772–782. doi: 10.1021/bi00880a023. [DOI] [PubMed] [Google Scholar]
- Sigman D. S., Mooser G. Chemical studies of enzyme active sites. Annu Rev Biochem. 1975;44:899–931. doi: 10.1146/annurev.bi.44.070175.004325. [DOI] [PubMed] [Google Scholar]
- Soon C. Y., Shepherd M. G., Sullivan P. A. Modification of lactate oxidase with diethyl pyrocarbonate. Evidence for an active-site histidine residue. Biochem J. 1977 Aug 1;165(2):385–393. doi: 10.1042/bj1650385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streamer M., Eriksson K. E., Pettersson B. Extracellular enzyme system utilized by the fungus Sporotrichum pulverulentum (Chrysosporium lignorum) for the breakdown of cullulose. Functional characterization of five endo-1,4-beta-glucanases and one exo-1,4-beta-glucanase. Eur J Biochem. 1975 Nov 15;59(2):607–613. doi: 10.1111/j.1432-1033.1975.tb02489.x. [DOI] [PubMed] [Google Scholar]


