Abstract
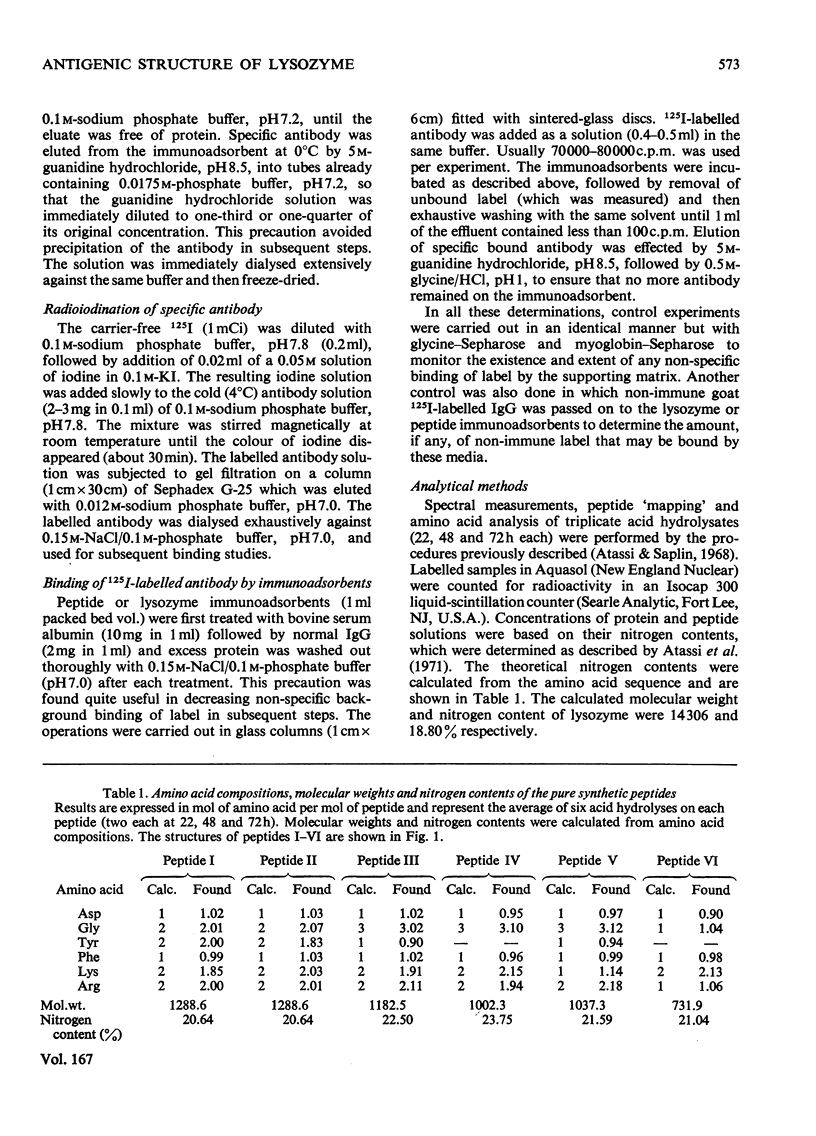
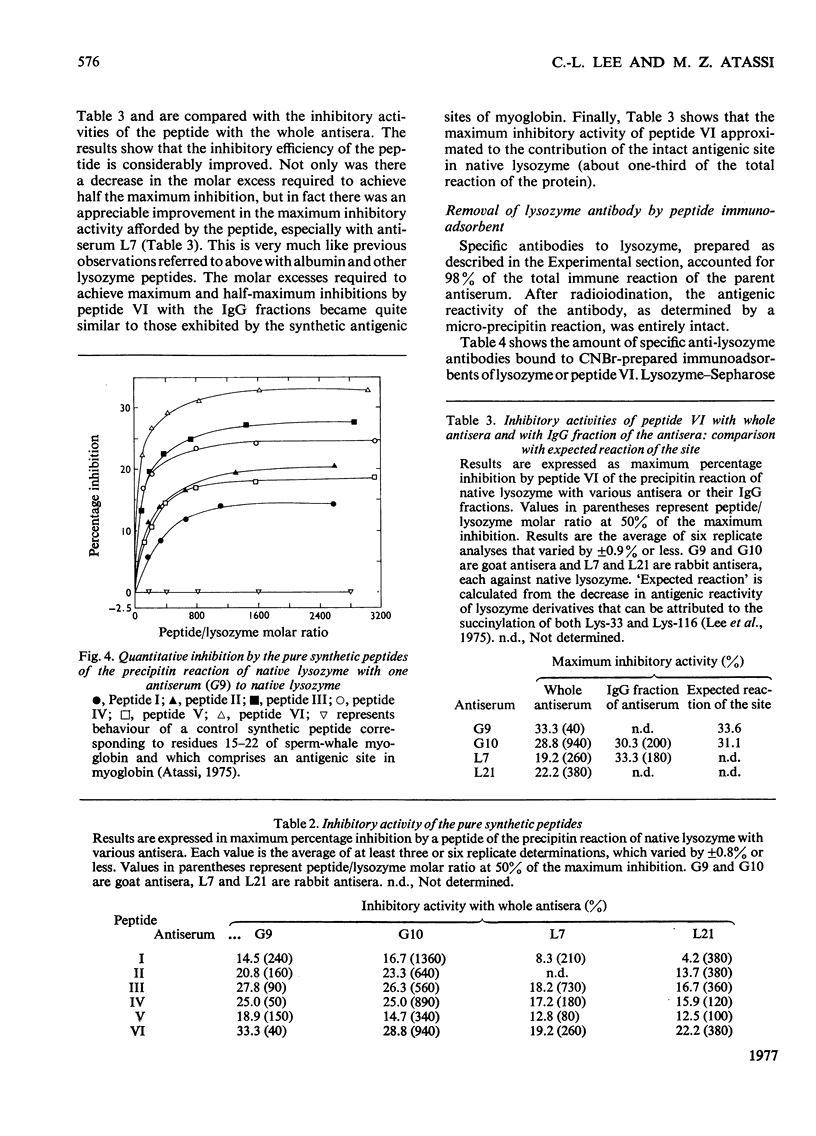
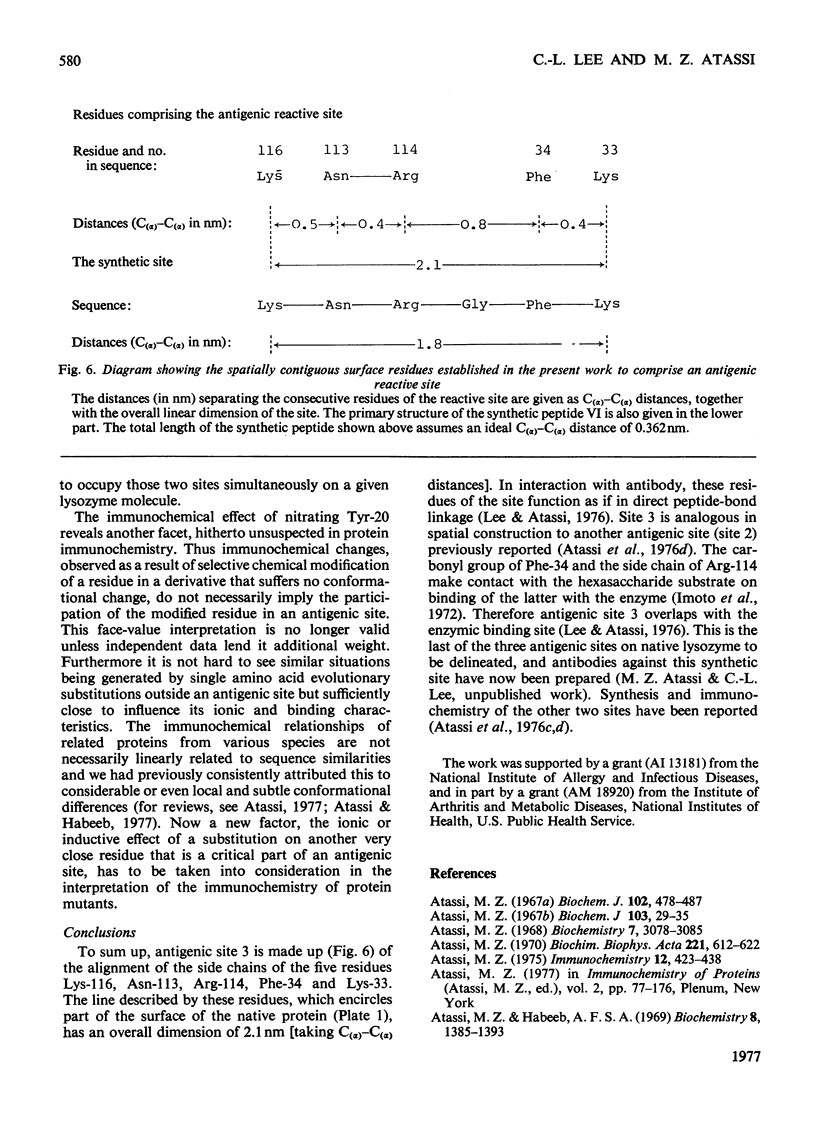
1. Previous reports from this laboratory have shown that both Lys-33 and Lys-116 are parts of an antigenic site in native lysozyme. Similar studies of tyrosine derivatives indicated that one or both of Tyr-20 and Tyr-23 are located in or very close to an antigenic site in lysozyme. The site, which was located around the disulphide bridge 30–115, was recently shown unequivocally to include the residues Tyr-20, Arg-21, Lys-116, Asn-113, Arg-114, Phe-34 and Lys-33. This was confirmed by the `surface-simulation' synthetic approach that we have recently developed, in which the foregoing eight surface residues were directly linked via peptide bonds, with intervening spacers where appropriate, into a single peptide. The peptide does not exist in native lysozyme, but simulates a surface region of it. 2. In the present work several surface-simulation peptides were synthesized representing various parts of the region, to determine the minimum structural feature that retains full antigenic reactivity and to investigate if the spatially constructed antigenic site has a preferred direction. 3. The peptide Lys-Asn-Arg-Gly-Phe-Lys exhibited a remarkable inhibitory activity towards the immune reaction of lysozyme and accounted entirely for the maximum expected reactivity of the site in the native protein (i.e. about one-third of the total lysozyme reactivity). An immunoadsorbent of the peptide bound about one-third of the total antibody to lysozyme. 4. The residues Tyr-20 and Arg-21 are not part of the site. The previously reported immunochemical effect observed on nitration of Tyr-20 was due to a deleterious ionic effect exerted by the modified tyrosine residue on the adjacent Lys-96, which is in an entirely different antigenic site of lysozyme. Thus the modification of Tyr-20 impairs the reactivity of an adjacent antigenic site, even though the residue itself is not part of a site. The conformational and immunochemical implications of this finding are discussed. 5. The antigenic site therefore comprises the five spatially adjacent residues Lys-116, Asn-113, Arg-114, Phe-34, Lys-33. The antigenic site exhibited a preferred direction (Lys-116 to Lys-33), since the reverse surface-simulation synthetic sequence was immunochemically inefficient. The site describes a line which circumscribes part [2.1nm in C(α)–C(α) distance from Lys-116 to Lys-33] of the surface of the molecule.
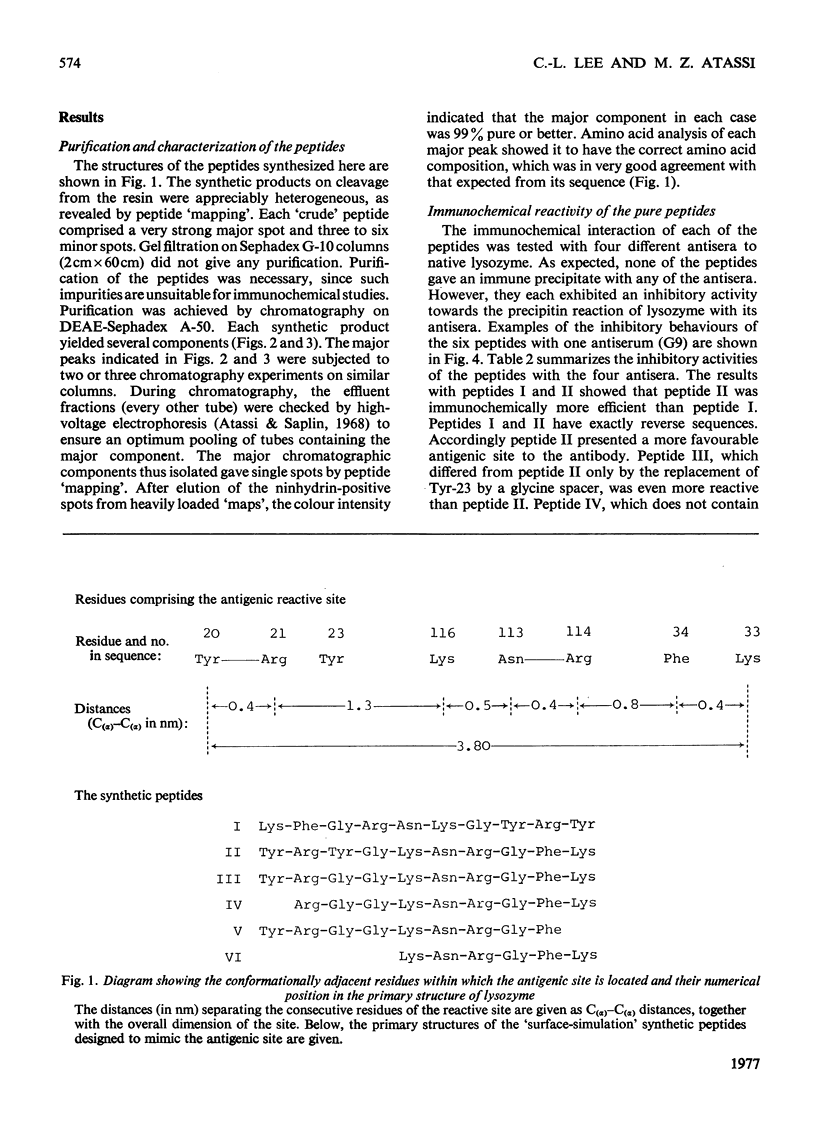
Full text
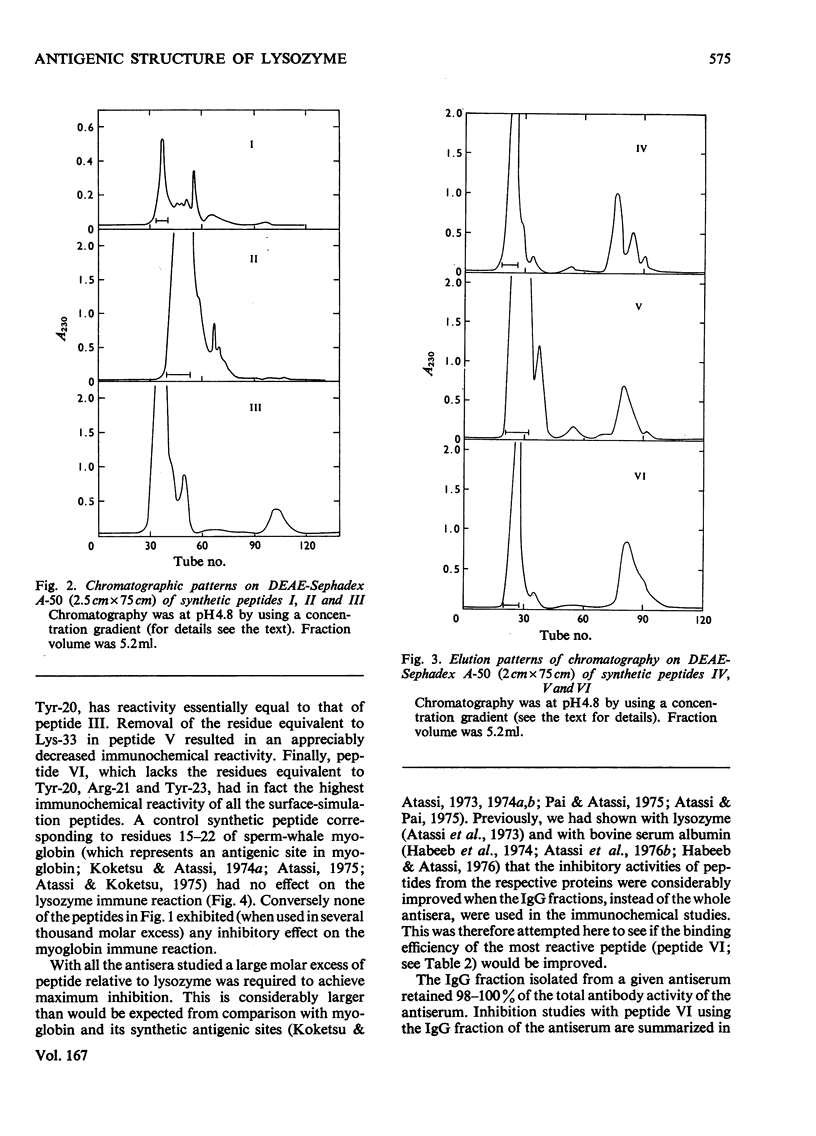
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atassi M. Z. Antigenic structure of myoglobin: the complete immunochemical anatomy of a protein and conclusions relating to antigenic structures of proteins. Immunochemistry. 1975 May;12(5):423–438. doi: 10.1016/0019-2791(75)90010-5. [DOI] [PubMed] [Google Scholar]
- Atassi M. Z., Habeeb A. F., Ando K. Enzymic and immunochemical properties of lysozyme. VII. Location of all the antigenic reactive regions. A new approach to study immunochemistry of tight proteins. Biochim Biophys Acta. 1973 Mar 23;303(1):203–209. [PubMed] [Google Scholar]
- Atassi M. Z., Habeeb A. F. Enzymic and immunochemical properties of lysozyme. I. Derivatives modified at tyrosine. Influence of nature of modification on activity. Biochemistry. 1969 Apr;8(4):1385–1393. doi: 10.1021/bi00832a012. [DOI] [PubMed] [Google Scholar]
- Atassi M. Z., Habeeb A. F., Lee C. L. Immunochemistry of serum albumin--II. Isolation and characterization of a fragment from the first third of bovine serum albumin carrying almost all the antigenic reactivity of the protein. Immunochemistry. 1976 Jun;13(6):547–555. doi: 10.1016/0019-2791(76)90332-3. [DOI] [PubMed] [Google Scholar]
- Atassi M. Z. Immunochemistry of sperm whale myoglobin. 3. Modification of the three tyrosine residues and their role in the conformation and differentiation of their roles in the antigenic reactivity. Biochemistry. 1968 Sep;7(9):3078–3085. doi: 10.1021/bi00849a008. [DOI] [PubMed] [Google Scholar]
- Atassi M. Z. Immunochemistry of sperm whale myoglobin. VI. Preparation and conformational analysis of eight mammalian myoglobins. Biochim Biophys Acta. 1970 Dec 22;221(3):612–622. doi: 10.1016/0005-2795(70)90233-3. [DOI] [PubMed] [Google Scholar]
- Atassi M. Z. Immunochemistry of sperm-whale myoglobins prepared with various modified porphyrins and metalloporphyrins. Biochem J. 1967 Apr;103(1):29–35. doi: 10.1042/bj1030029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atassi M. Z., Koketsu J., Habeeb A. F. Enzymic and immunochemical properties of lysozyme. XIII. Accurate delineation of the reactive site around the disulfide 6-127 by immunochemical study of beta-propiolactone lysozyme derivative and of synthetic disulfide peptides. Biochim Biophys Acta. 1976 Feb 20;420(2):358–375. doi: 10.1016/0005-2795(76)90328-7. [DOI] [PubMed] [Google Scholar]
- Atassi M. Z., Koketsu J. Immunochemistry of sperm-whale myoglobin--XXIII. Investigation of the independence of the five antigenic reactive regions by immunoabsorbent studies. Immunochemistry. 1975 Sep;12(9):741–744. [PubMed] [Google Scholar]
- Atassi M. Z., Lee C. L., Pai R. C. Enzymic and immunochemical properties of lysozyme. XVI. A novel synthetic approach to an antigenic reactive site by direct linkage of the relevant conformationally adjacent residues constituting the site. Biochim Biophys Acta. 1976 Apr 14;427(2):745–751. doi: 10.1016/0005-2795(76)90219-1. [DOI] [PubMed] [Google Scholar]
- Atassi M. Z., Li Lee C. Enzymic and immunochemical properties of lysozyme-XII. Delineation of the reactive site around the two central disulfides by immunochemical and conformational studies of derivatives of the two-disulfide peptide. Immunochemistry. 1976 Jan;13(1):7–14. doi: 10.1016/0019-2791(76)90290-1. [DOI] [PubMed] [Google Scholar]
- Atassi M. Z., Litowich M. T., Andres S. F. Immunochemistry of sperm-whale myoglobin--XXI. Conformation and immunochemistry of derivatives modified at certain histidine residues. Immunochemistry. 1975 Sep;12(9):727–733. [PubMed] [Google Scholar]
- Atassi M. Z., Pai R. C. Immunochemistry of sperm-whale myoglobin--XXII. Accurate delineation of the single reactive region in sequence 103-120 by immunochemical studies of synthetic peptides: the complete antigenic structure of the protein. Immunochemistry. 1975 Sep;12(9):735–740. [PubMed] [Google Scholar]
- Atassi M. Z. Periodate oxidation of sperm-whale myoglobin and the role of the methionine residues in the antigen-antibody reaction. Biochem J. 1967 Feb;102(2):478–487. doi: 10.1042/bj1020478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atassi M. Z., Perlstein M. T., Habeeb A. F. Conformational studies on modified proteins and peptides. IV. Conformation of lysozyme derivatives modified at tyrosine or at tryptophan residues. J Biol Chem. 1971 May 25;246(10):3291–3296. [PubMed] [Google Scholar]
- Atassi M. Z., Saplin B. J. Immunochemistry of sperm whale myoglobin. I. The specific interaction of some tryptic peptides and of peptides containing all the reactive regions of the antigen. Biochemistry. 1968 Feb;7(2):688–698. doi: 10.1021/bi00842a026. [DOI] [PubMed] [Google Scholar]
- Atassi M. Z., Thomas A. V. Immunochemistry of sperm whale myoglobin. IV. The role of the arginine residues in the conformation and differentiation of their roles in the antigenic reactivity. Biochemistry. 1969 Aug;8(8):3385–3394. doi: 10.1021/bi00836a037. [DOI] [PubMed] [Google Scholar]
- Blake C. C., Mair G. A., North A. C., Phillips D. C., Sarma V. R. On the conformation of the hen egg-white lysozyme molecule. Proc R Soc Lond B Biol Sci. 1967 Apr 18;167(1009):365–377. doi: 10.1098/rspb.1967.0034. [DOI] [PubMed] [Google Scholar]
- Brandt J., Andersson L. O., Porath J. Covalent attachment of proteins to polysaccharide carriers by means of benzoquinone. Biochim Biophys Acta. 1975 Mar 28;386(1):196–202. doi: 10.1016/0005-2795(75)90259-7. [DOI] [PubMed] [Google Scholar]
- Habeeb A. F., Atassi M. Z. A fragment comprising the last third of bovine serum albumin which accounts for almost all the antigenic reactivity of the native protein. J Biol Chem. 1976 Aug 10;251(15):4616–4621. [PubMed] [Google Scholar]
- Habeeb A. F., Atassi M. Z. Enzymic and immunochemical properties of lysozyme. IV. Demonstration of conformational differences between alpha-lactalbumin and lysozyme. Biochim Biophys Acta. 1971 Apr 27;236(1):131–141. doi: 10.1016/0005-2795(71)90158-9. [DOI] [PubMed] [Google Scholar]
- Habeeb A. F., Atassi M. Z., Lee C. L. Peptides with strong immunochemical inhibitory activity from bovine serum albumin. Application of a novel approach. Biochim Biophys Acta. 1974 Apr 11;342(2):389–395. doi: 10.1016/0005-2795(74)90097-x. [DOI] [PubMed] [Google Scholar]
- Koketsu J., Atassi M. Z. Immunochemistry of sperm-whale myoglobin. 18. Accurate delineation of the single reactive region in sequence 120-153 by study of synthetic peptides. Biochim Biophys Acta. 1973 Dec 6;328(2):289–302. [PubMed] [Google Scholar]
- Koketsu J., Atassi M. Z. Immunochemistry of sperm-whale myoglobin. XIX. Accurate delineation of the single reactive region in sequence 54-85 by immunochemical study of synthetic peptides. Biochim Biophys Acta. 1974 Mar 14;342(1):21–29. [PubMed] [Google Scholar]
- Koketsu J., Atassi M. Z. Immunochemistry of sperm-whale myoglobin. XVI. Accurate delineation of the single region in sequence 1-55 by immunochemical studies of synthetic peptides. Some conclusions concerning antigenic structures of proteins. Immunochemistry. 1974 Jan;11(1):1–8. doi: 10.1016/0019-2791(74)90335-8. [DOI] [PubMed] [Google Scholar]
- Lee C. L., Atassi M. Z. Delineation of the third antigenic site of lysozyme by application of a novel 'surface-simulation' synthetic approach directly linking the conformationally adjacent residues forming the site. Biochem J. 1976 Oct 1;159(1):89–93. doi: 10.1042/bj1590089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. L., Atassi M. Z. Enzymic and immunochemical properties of lysozyme. XI. Conformation and immunochemistry of the two-disulfide peptide and the tryptophan and lysine residues in its antigenic reactivity. Biochim Biophys Acta. 1975 Oct 20;405(2):464–474. [PubMed] [Google Scholar]
- Lee C. L., Pai R. C., Atassi M. Z. Enzymic and immunochemical properties of lysozyme--XV. Delineation of the reactive site around the two central disulfides by immunochemical studies of novel synthetic peptides that contain diglycyl bridges instead of disulfides. Immunochemistry. 1976 Aug;13(8):681–687. doi: 10.1016/0019-2791(76)90209-3. [DOI] [PubMed] [Google Scholar]
- Li Lee C., Atassi M. Z., Habeeb A. F. Enzymic and immunochemical properties of lysozyme. IX. Conformation and immunochemistry of derivatives succinylated at certain lysine residues. Biochim Biophys Acta. 1975 Aug 19;400(2):423–432. doi: 10.1016/0005-2795(75)90198-1. [DOI] [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- Pai R. C., Atassi M. Z. Immunochemistry of sperm-whale myoglobin--XX. Accurate delineation of the single reactive region in sequence 80-103 by immunochemical studies of synthetic peptides. Immunochemistry. 1975 Apr;12(4):285–290. doi: 10.1016/0019-2791(75)90177-9. [DOI] [PubMed] [Google Scholar]
- Sokolovsky M., Riordan J. F., Vallee B. L. Conversion of 3-nitrotyrosine to 3-aminotyrosine in peptides and proteins. Biochem Biophys Res Commun. 1967 Apr 7;27(1):20–25. doi: 10.1016/s0006-291x(67)80033-0. [DOI] [PubMed] [Google Scholar]



