Abstract
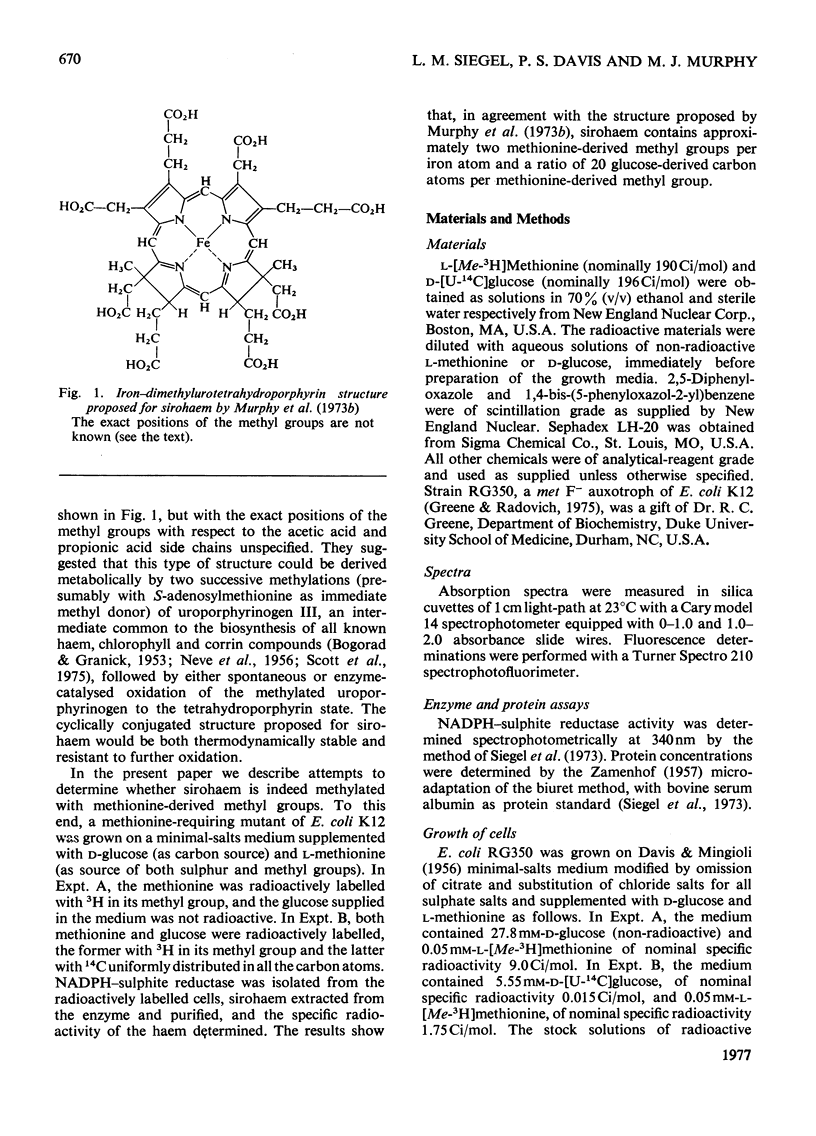
Sirohaem is a new type of haem that has been detected as a prosthetic group of several bacterial and plant enzymes that catalyse the six-electron reductions of sulphite to sulphide or of nitrite to NH3. When a methionine-requiring mutant of Escherichia coli K12 was grown on a minimal medium supplemented with d-glucose and l-[Me-3H]methionine, 2.4 methyl groups per spectrophotometrically detectable haem group were incorporated into the sirohaem prosthetic group of the NADPH–sulphite reductase isolated from the organism. When the same strain of cells was grown on minimal medium supplemented with d-[U-14C]glucose and l-[Me-3H]methionine, the sirohaem isolated was found to contain a ratio of glucose-derived carbon/methionine-derived methyl of 19.8. This ratio is in excellent agreement with the value of 20 predicted by the iron–dimethyl-urotetrahydroporphyrin structure for sirohaem proposed by Murphy, Siegel, Kamin & Rosenthal [(1973) J. Biol. Chem. 248, 2801–2814]. It can be concluded that sirohaem is indeed methylated, with the methyl groups derived from methionine (rather than by modification of existing side chains, as in protohaem). The structure proposed by Murphy et al. (1973) is therefore probably correct in its essential features. A possible relationship between the pathway for biosynthesis of sirohaem and that for synthesis of vitamin B12 is discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOGORAD L., GRANICK S. Protoporphyrin precursors produced by a Chlorella mutant. J Biol Chem. 1953 Jun;202(2):793–800. [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene R. C., Radovich C. Role of methionine in the regulation of serine hydroxymethyltransferase in Eschericia coli. J Bacteriol. 1975 Oct;124(1):269–278. doi: 10.1128/jb.124.1.269-278.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAZZARINI R. A., ATKINSON D. E. A triphosphopyridine nucleotide-specific nitrite reductase from Escherichia coli. J Biol Chem. 1961 Dec;236:3330–3335. [PubMed] [Google Scholar]
- Murphy M. J., Siegel L. M., Kamin H., DerVartanian D. V., Lee J. P., LeGall J., Peck H. D., Jr An iron tetrahydroporphyrin prosthetic group common to both assimilatory and dissimilatory sulfite reductases. Biochem Biophys Res Commun. 1973 Sep 5;54(1):82–88. doi: 10.1016/0006-291x(73)90891-7. [DOI] [PubMed] [Google Scholar]
- Murphy M. J., Siegel L. M., Kamin H., Rosenthal D. Reduced nicotinamide adenine dinucleotide phosphate-sulfite reductase of enterobacteria. II. Identification of a new class of heme prosthetic group: an iron-tetrahydroporphyrin (isobacteriochlorin type) with eight carboxylic acid groups. J Biol Chem. 1973 Apr 25;248(8):2801–2814. [PubMed] [Google Scholar]
- Murphy M. J., Siegel L. M. Siroheme and sirohydrochlorin. The basis for a new type of porphyrin-related prosthetic group common to both assimilatory and dissimilatory sulfite reductases. J Biol Chem. 1973 Oct 10;248(19):6911–6919. [PubMed] [Google Scholar]
- Murphy M. J., Siegel L. M., Tove S. R., Kamin H. Siroheme: a new prosthetic group participating in six-electron reduction reactions catalyzed by both sulfite and nitrite reductases. Proc Natl Acad Sci U S A. 1974 Mar;71(3):612–616. doi: 10.1073/pnas.71.3.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- Scott I. A., Georgopapadakou N., Ho K. S., Klioze S., Lee E., Lee SL Temme G. H., Townsend C. A., Armitage I. M. Letter: Concerning the intermediacy of uro'gen III and of a heptacarboxylic uro'gen in corrinoid biosynthesis. J Am Chem Soc. 1975 Apr 30;97(9):2548–2550. doi: 10.1021/ja00842a045. [DOI] [PubMed] [Google Scholar]
- Siegel L. M., Davis P. S., Kamin H. Reduced nicotinamide adenine dinucleotide phosphate-sulfite reductase of enterobacteria. 3. The Escherichia coli hemoflavoprotein: catalytic parameters and the sequence of electron flow. J Biol Chem. 1974 Mar 10;249(5):1572–1586. [PubMed] [Google Scholar]
- Siegel L. M., Murphy M. J., Kamin H. Reduced nicotinamide adenine dinucleotide phosphate-sulfite reductase of enterobacteria. I. The Escherichia coli hemoflavoprotein: molecular parameters and prosthetic groups. J Biol Chem. 1973 Jan 10;248(1):251–264. [PubMed] [Google Scholar]
- Vega J. M., Garrett R. H. Siroheme: a prosthetic group of the Neurospora crassa assimilatory nitrite reductase. J Biol Chem. 1975 Oct 25;250(20):7980–7989. [PubMed] [Google Scholar]


