Abstract
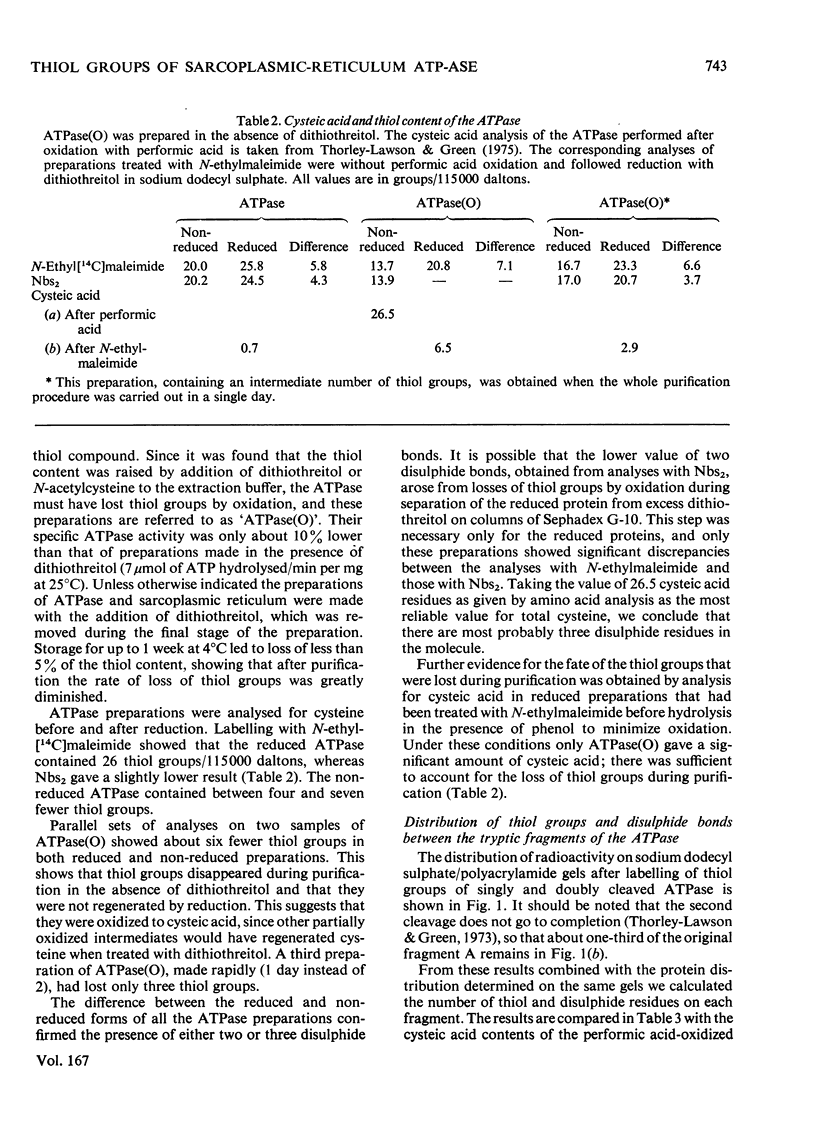
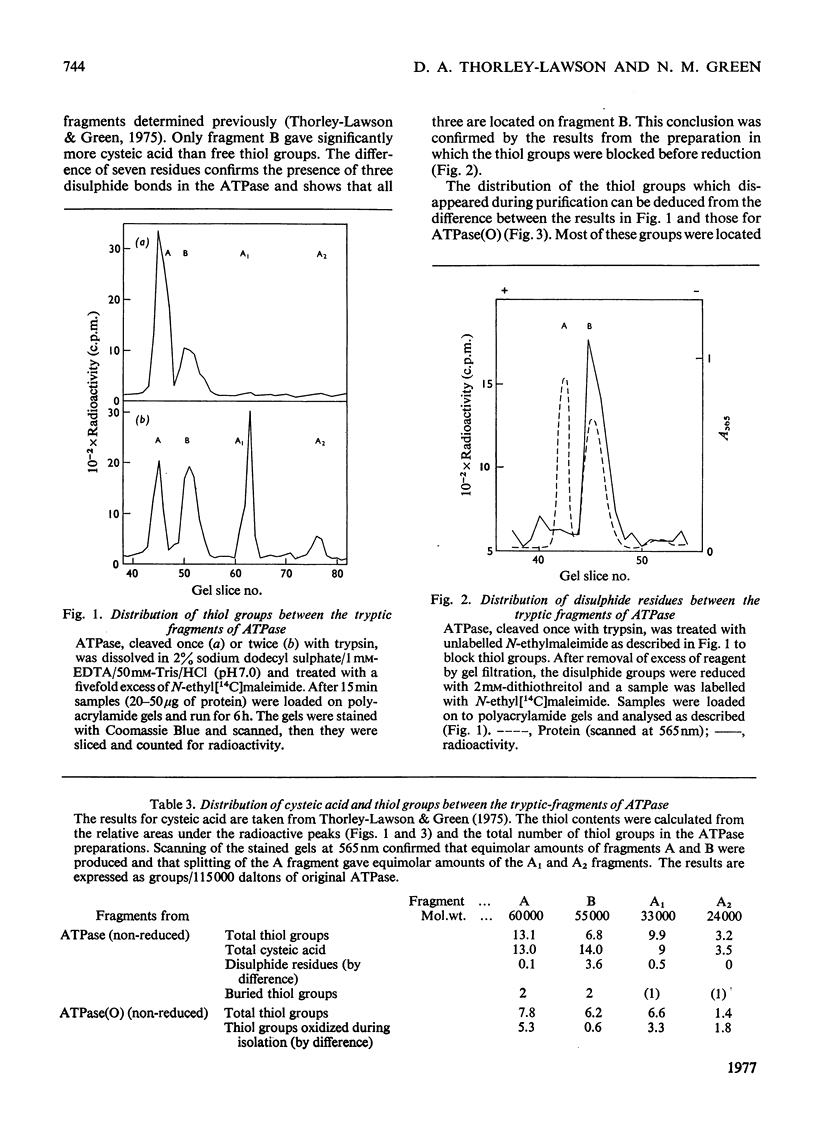
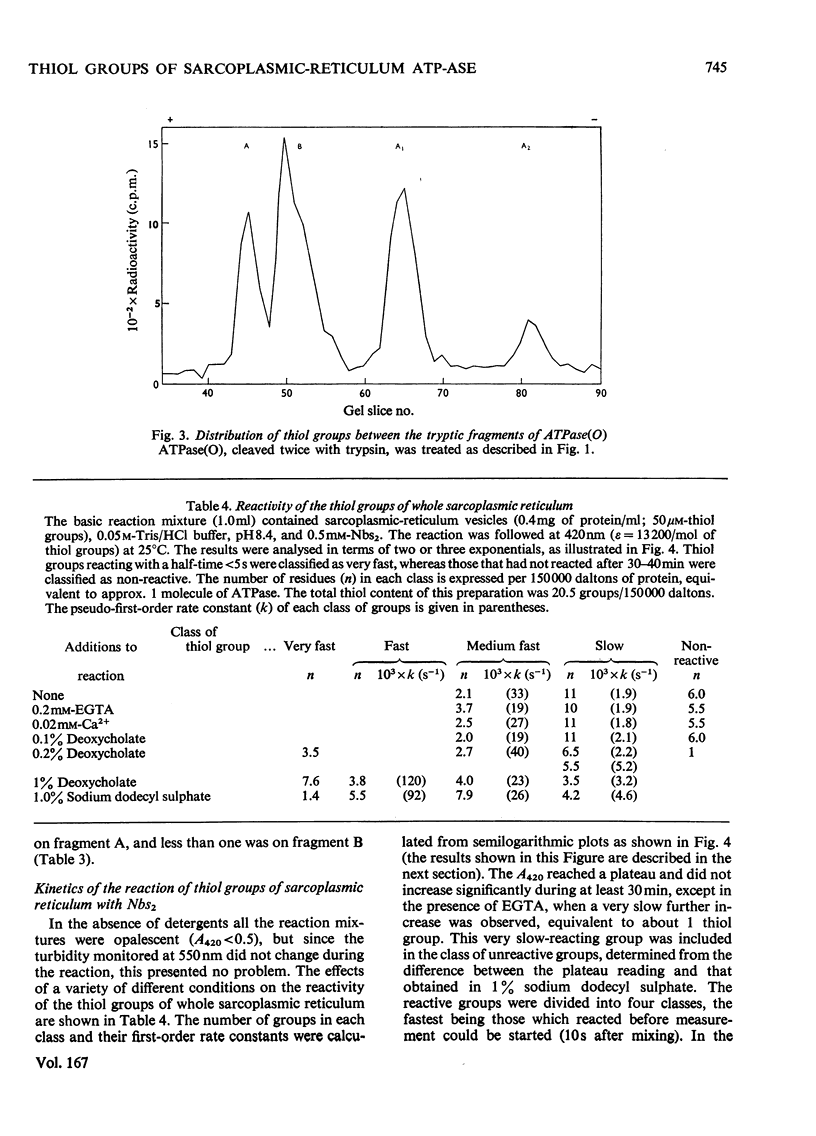
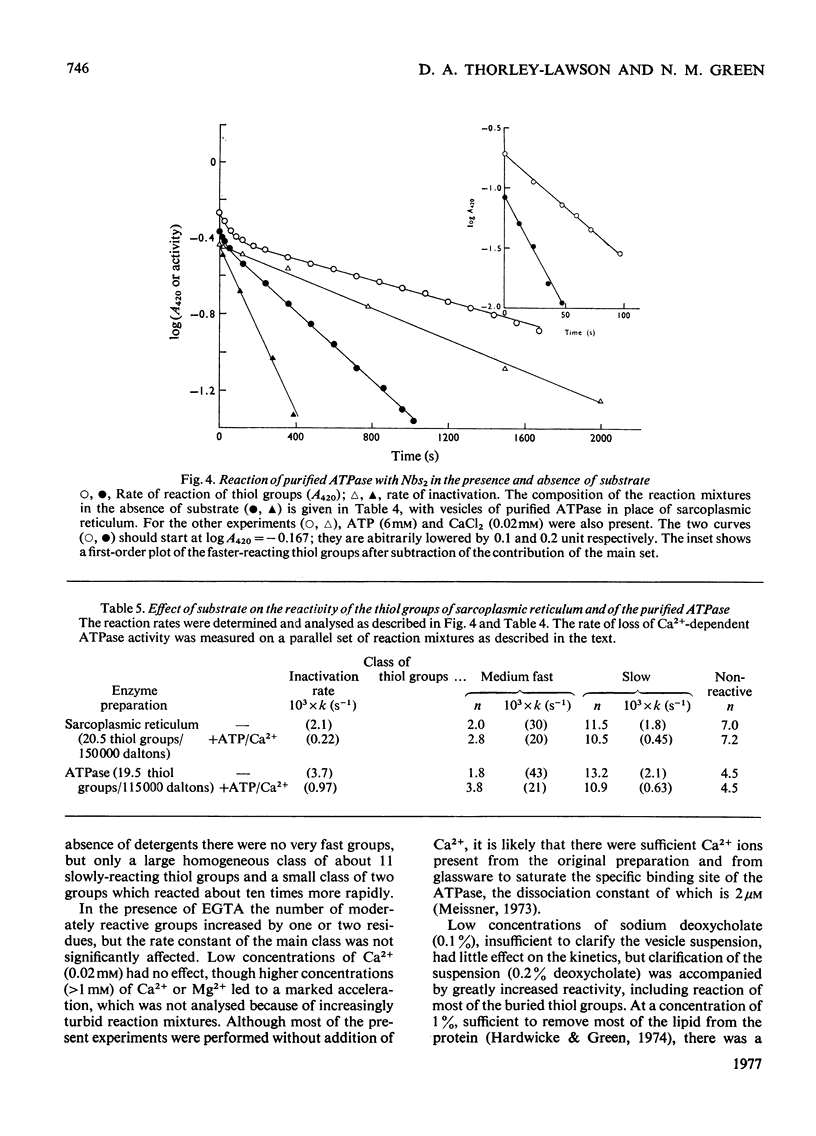
The ATPase (adenosine triphosphatase) from sarcoplasmic reticulum contains 20 thiol groups/115000 daltons, measured by using either N-ethyl[14C]maleimide or 5,5′-dithiobis-(2-nitrobenzoate) in sodium dodecyl sulphate. After reduction there were 26 thiol groups, in good agreement with 26.5 residues of cysteic acid found by amino acid analysis. The difference between this and the 20 residues measured before reduction implies the presence of three disulphide residues. The same number of disulphide residues was found by direct measurement. Three to six fewer thiol groups were found in preparations made in the absence of dithiothreitol. The missing residues were accounted for as cysteic acid. The distribution of disulphide bonds and of exposed and buried thiol groups among the tryptic fragments of the molecule was measured after labelling with N-ethyl[14C]-maleimide. The disulphides were confined to fragment B (mol.wt. 55000), whereas several thiol groups were present on each of the fragments (A, B, A1 and A2). The kinetics of the reaction of the ATPase with 5,5′-dithiobis-(2-nitrobenzoate) showed that four or five of the thiol groups were unreactive in the absence of detergent and that 13 of the remainder reacted with a single first-order rate constant. In the presence of ATP and Ca2+ the reaction rate of all but two groups of this class was uniformly decreased. In the presence or absence of ATP and Ca2+ the rate constant for inactivation was close to the rate constant for this class, but was not identical with it. No selective protection of a specific active-site-thiol group was observed. Parallel experiments with sarcoplasmic reticulum gave similar results, except that the reaction rates were a little lower and there were two more buried groups. Solution of ATPase of sarcoplasmic reticulum in detergent greatly increased the reactivity of all thiol groups. The effects of low concentrations of deoxycholate were reversible. EGTA or low concentrations (0.02mm) of Ca2+ of Mg2+ had very little effect on the reactivity.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen G., Green N. M. A 31-residue tryptic peptide from the active site of the [Ca++]-transporting adenosine triphosphatase of rabbit sarcoplasmic reticulum. FEBS Lett. 1976 Mar 15;63(1):188–192. doi: 10.1016/0014-5793(76)80223-2. [DOI] [PubMed] [Google Scholar]
- Dulley J. R., Grieve P. A. A simple technique for eliminating interference by detergents in the Lowry method of protein determination. Anal Biochem. 1975 Mar;64(1):136–141. doi: 10.1016/0003-2697(75)90415-7. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- GOA J. A micro biuret method for protein determination; determination of total protein in cerebrospinal fluid. Scand J Clin Lab Invest. 1953;5(3):218–222. doi: 10.3109/00365515309094189. [DOI] [PubMed] [Google Scholar]
- Hardwicke P. M., Green N. M. The effect of delipidation on the adenosine triphosphatase of sarcoplasmic reticulum. Electron microscopy and physical properties. Eur J Biochem. 1974 Feb 15;42(1):183–193. doi: 10.1111/j.1432-1033.1974.tb03328.x. [DOI] [PubMed] [Google Scholar]
- Inesi G., Maring E., Murphy A. J., McFarland B. H. A study of the phosphorylated intermediate of sarcoplasmic reticulum ATPase. Arch Biochem Biophys. 1970 May;138(1):285–294. doi: 10.1016/0003-9861(70)90309-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Little C., O'Brien P. J. Products of oxidation of a protein thiol group after reaction with various oxidizing agents. Arch Biochem Biophys. 1967 Nov;122(2):406–410. doi: 10.1016/0003-9861(67)90212-3. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis C., Shooter E. M. The proteins of rabbit skeletal muscle sarcoplasmic reticulum. Arch Biochem Biophys. 1972 Dec;153(2):641–655. doi: 10.1016/0003-9861(72)90383-9. [DOI] [PubMed] [Google Scholar]
- MacLennan D. H. Purification and properties of an adenosine triphosphatase from sarcoplasmic reticulum. J Biol Chem. 1970 Sep 10;245(17):4508–4518. [PubMed] [Google Scholar]
- MacLennan D. H., Seeman P., Iles G. H., Yip C. C. Membrane formation by the adenosine triphosphatase of sarcoplasmic reticulum. J Biol Chem. 1971 Apr 25;246(8):2702–2710. [PubMed] [Google Scholar]
- Martonosi A. Sarcoplasmic reticulum. IV. Solubilization of microsomal adenosine triphosphatase. J Biol Chem. 1968 Jan 10;243(1):71–81. [PubMed] [Google Scholar]
- Martonosi A. The effect of ATP upon the reactivity of SH groups in sarcoplasmic reticulum membranes. FEBS Lett. 1976 Aug 15;67(2):153–155. doi: 10.1016/0014-5793(76)80354-7. [DOI] [PubMed] [Google Scholar]
- Meissner G. ATP and Ca2+ binding by the Ca2+ pump protein of sarcoplasmic reticulum. Biochim Biophys Acta. 1973 Apr 16;298(4):906–926. doi: 10.1016/0005-2736(73)90395-7. [DOI] [PubMed] [Google Scholar]
- Meissner G., Fleischer S. Dissociation and reconstitution of functional sarcoplasmic reticulum vesicles. J Biol Chem. 1974 Jan 10;249(1):302–309. [PubMed] [Google Scholar]
- Migala A., Agostini B., Hasselbach W. Tryptic fragmentation of the calcium transport system in the sarcoplasmic reticulum. Z Naturforsch C. 1973 Mar-Apr;28(3):178–182. doi: 10.1515/znc-1973-3-414. [DOI] [PubMed] [Google Scholar]
- Murphy A. J. Sulfhydryl group modification of sarcoplasmic reticulum membranes. Biochemistry. 1976 Oct 5;15(20):4492–4496. doi: 10.1021/bi00665a025. [DOI] [PubMed] [Google Scholar]
- Neet K. E., Green N. M. Kinetics of the cooperativity of the Ca2+-transporting adenosine triphosphatase of sarcoplasmic reticulum and the mechanism of the ATP interaction. Arch Biochem Biophys. 1977 Jan 30;178(2):588–597. doi: 10.1016/0003-9861(77)90230-2. [DOI] [PubMed] [Google Scholar]
- Panet R., Selinger Z. Specific alkylation of the sarcoplasmic reticulum ATPase by N-ethyl-[1-14C]maleimide and identification of the labeled protein in acrylamide gel-electrophoresis. Eur J Biochem. 1970 Jul;14(3):440–444. doi: 10.1111/j.1432-1033.1970.tb00308.x. [DOI] [PubMed] [Google Scholar]
- Rizzolo L. J., Maire M., Reynolds J. A., Tanford C. Molecular weights and hydrophobicity of the polypeptide chain of sarcoplasmic reticulum calcium(II) adenosine triphosphatase and of its primary tryptic fragments. Biochemistry. 1976 Aug 10;15(16):3433–3437. doi: 10.1021/bi00661a006. [DOI] [PubMed] [Google Scholar]
- Stewart P. S., MacLennan D. H. Isolation and characterization of tryptic fragments of the adenosine triphosphatase of sarcoplasmic reticulum. J Biol Chem. 1976 Feb 10;251(3):712–719. [PubMed] [Google Scholar]
- Stewart P. S., MacLennan D. H. Surface particles of sarcoplasmic reticulum membranes. Structural features of the adenosine triphosphatase. J Biol Chem. 1974 Feb 10;249(3):985–993. [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Green N. M. Separation and characterisation of tryptic fragments from the adenosine triphosphatase of sarcoplasmic reticulum. Eur J Biochem. 1975 Nov 1;59(1):193–200. doi: 10.1111/j.1432-1033.1975.tb02441.x. [DOI] [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Green N. M. Studies on the location and orientation of proteins in the sarcoplasmic reticulum. Eur J Biochem. 1973 Dec 17;40(2):403–413. doi: 10.1111/j.1432-1033.1973.tb03209.x. [DOI] [PubMed] [Google Scholar]
- le Maire M., Jorgensen K. E., Roigaard-Petersen H., Moller J. V. Properties of deoxycholate solubilized sarcoplasmic reticulum Ca2+-ATPase. Biochemistry. 1976 Dec 28;15(26):5805–5812. doi: 10.1021/bi00671a018. [DOI] [PubMed] [Google Scholar]


