Abstract
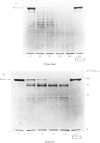
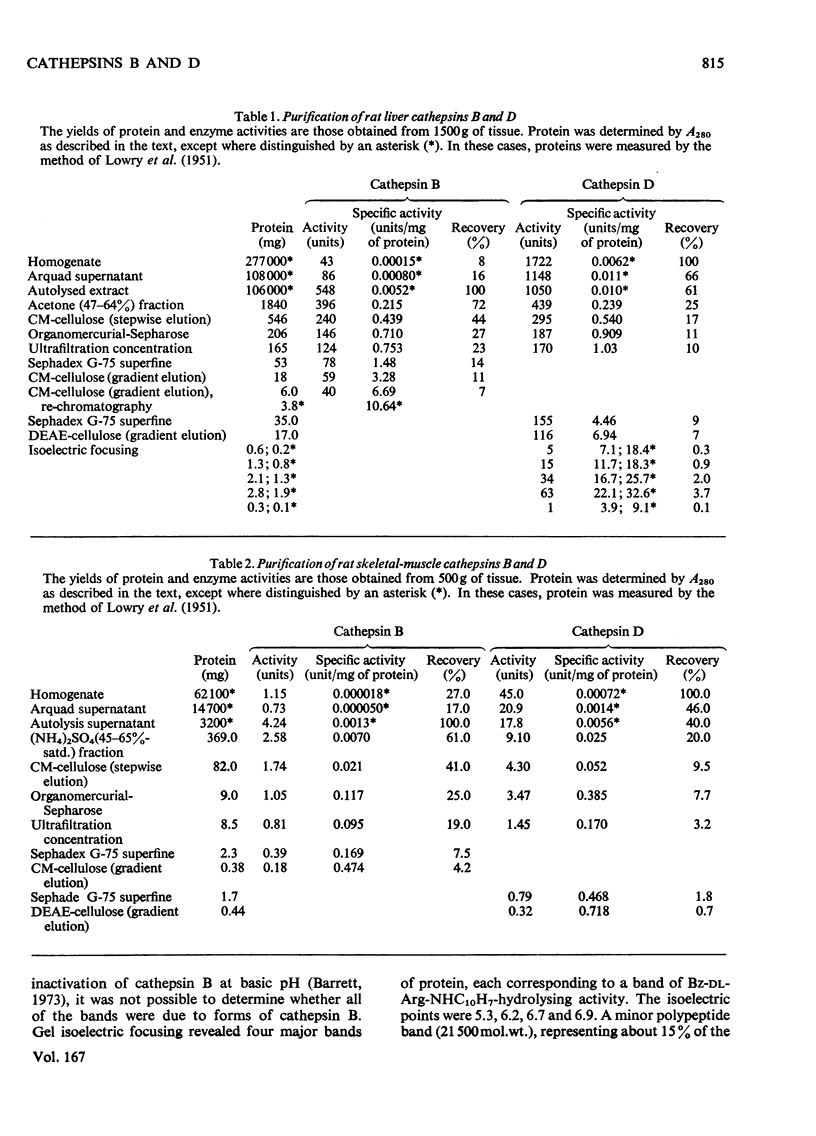
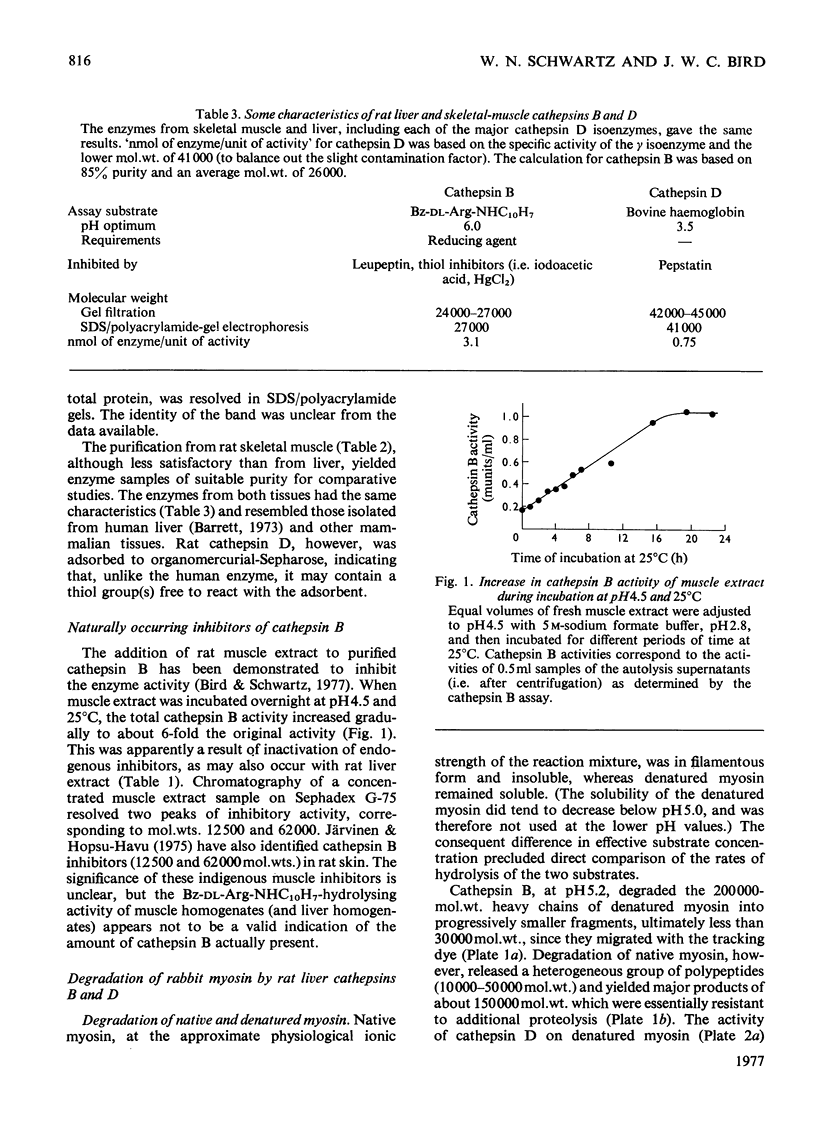
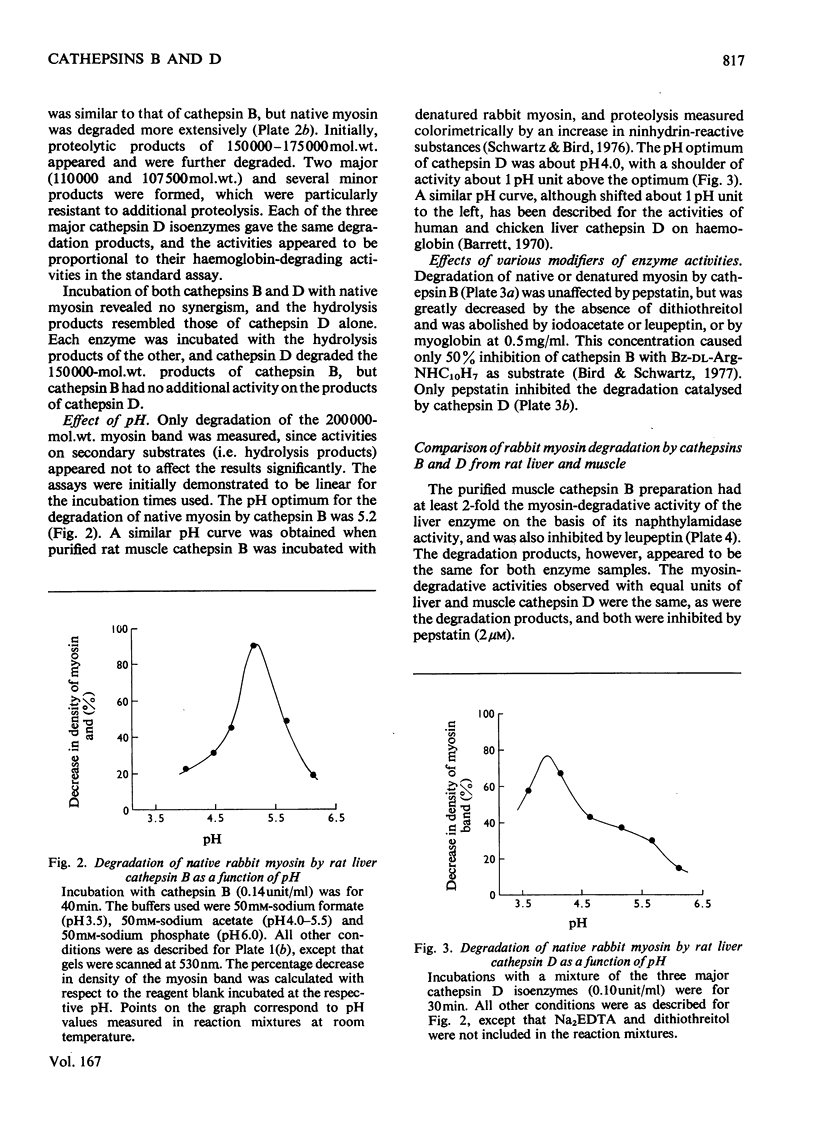
1. The procedure of Barrett [(1973) Biochem. J. 131, 809–822] for isolating cathepsins B and D from human liver was modified for use with rat liver and skeletal muscle. The purified enzymes appeared to be similar to those reported in other species. 2. Sephadex G-75 chromatography of concentrated muscle extract resolved two peaks of cathepsin B inhibitory activity, corresponding to molecular weights of 12500 and 62000. 3. The degradation of purified myofibrillar proteins by cathepsins B and D was clearly demonstrated by sodium dodecyl sulphate/polyacrylamide-gel electrophoresis. After incubation with enzyme, the polypeptide bands representing the substrates decreased in intensity and lower molecular weight products appeared. 4. Cathepsins B and D, purified from either rat liver or skeletal muscle, were shown to degrade myosin, purified from either rabbit or rat muscle. Soluble denatured myosin was degraded more extensively than insoluble native myosin. Degradation by cathepsin B was inhibited by lack of reducing agent, or by myoglobin, iodoacetic acid and leupeptin, but not by pepstatin. The same potential modifiers were applied to cathepsin D, and only pepstatin produced inhibition. 5. Rat liver cathepsin B had a pH optimum of 5.2 on native rabbit myosin. The pH optimum of cathepsin D was 4.0, with a shoulder of activity about 1pH unit above the optimum. 6. Rat liver cathepsins B and D were demonstrated to degrade rabbit F-actin at pH5.0, and were inhibited by leupeptin and pepstain, respectively. 7. The degradation of myosin and actin by cathepsin D was more extensive than that by cathepsin B.
Full text
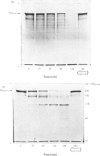
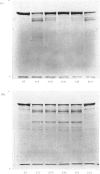
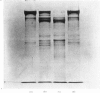
PDF



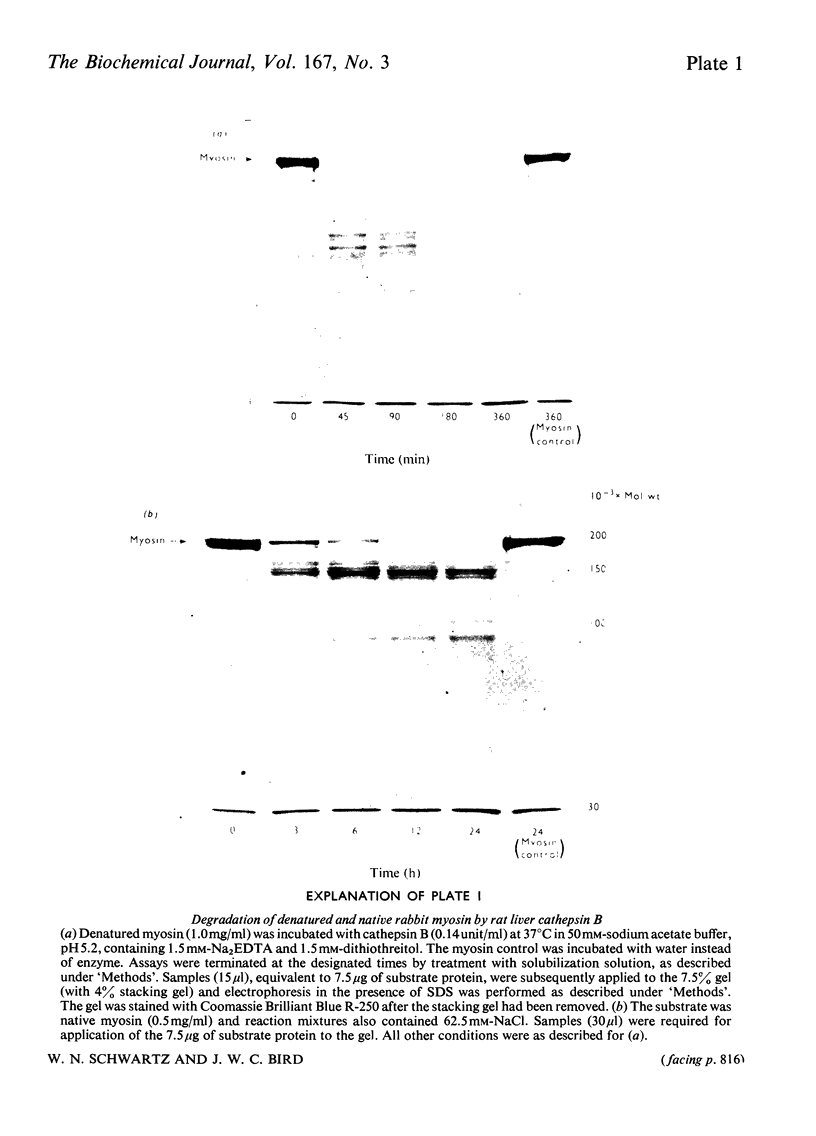

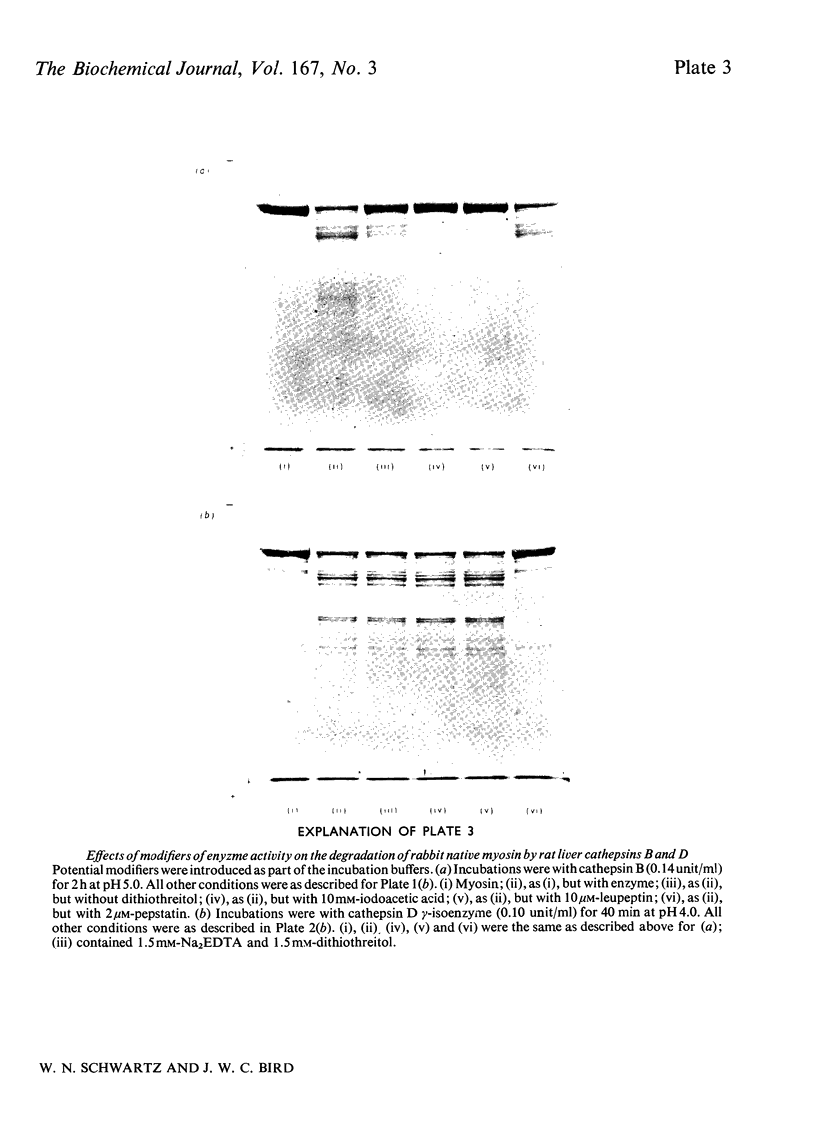
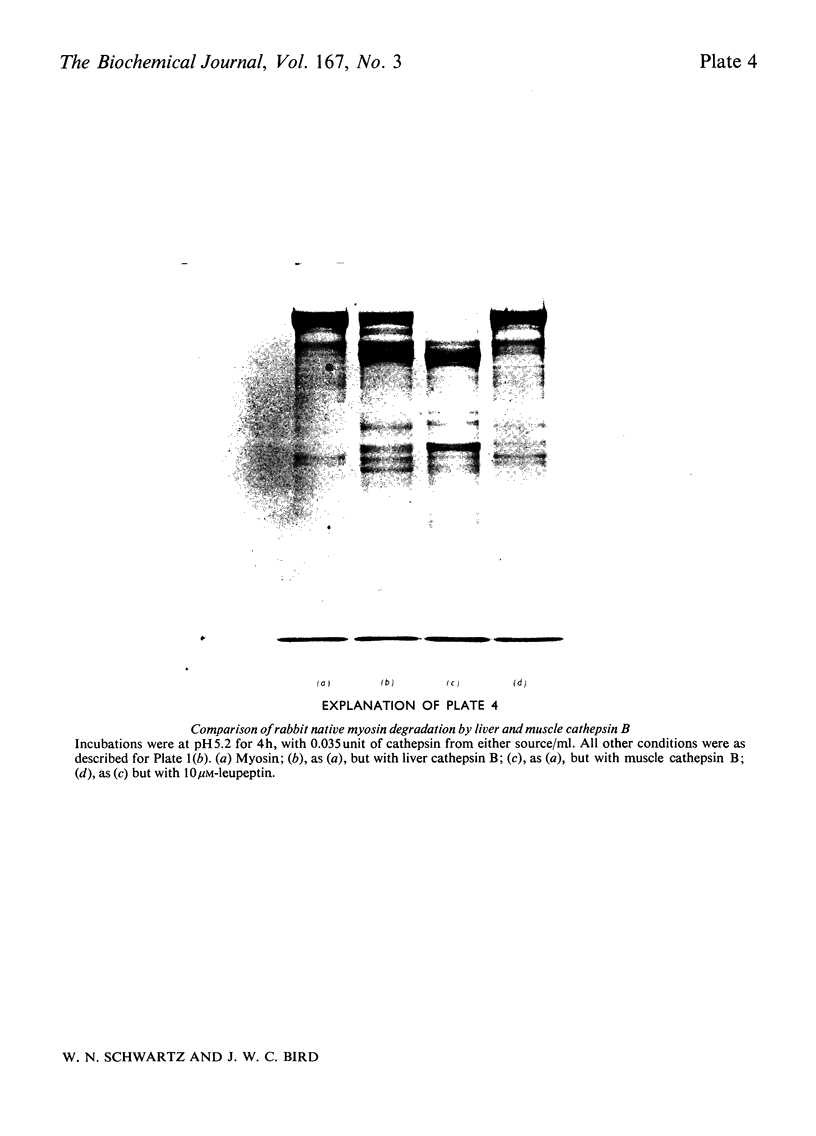

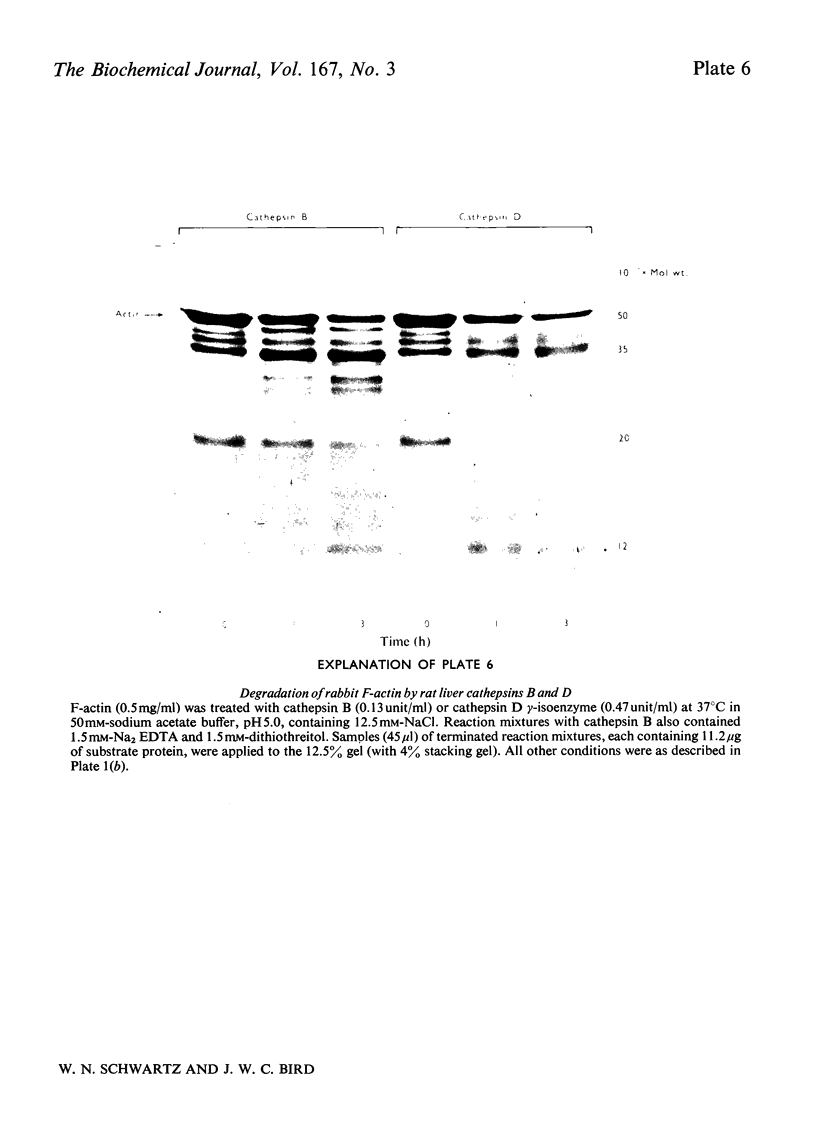



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett A. J. A new assay for cathepsin B1 and other thiol proteinases. Anal Biochem. 1972 May;47(1):280–293. doi: 10.1016/0003-2697(72)90302-8. [DOI] [PubMed] [Google Scholar]
- Barrett A. J. Cathepsin D. Purification of isoenzymes from human and chicken liver. Biochem J. 1970 Apr;117(3):601–607. doi: 10.1042/bj1170601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J. Human cathepsin B1. Purification and some properties of the enzyme. Biochem J. 1973 Apr;131(4):809–822. doi: 10.1042/bj1310809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch W. A., Stromer M. H., Goll D. E., Suzuki A. Ca 2+ -specific removal of Z lines from rabbit skeletal muscle. J Cell Biol. 1972 Feb;52(2):367–381. doi: 10.1083/jcb.52.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canonico P. G., Bird J. W. Lysosomes in skeletal muscle tissue. Zonal centrifugation evidence for multiple cellular sources. J Cell Biol. 1970 May;45(2):321–333. doi: 10.1083/jcb.45.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Albis A., Béchet J. J. Effet du pH sur la fixation d'inhibiteurs compétitifs synthétiques par la trypsine. Biochim Biophys Acta. 1967 Aug 15;140(3):435–458. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Davidson E., Poole B. Fractionation of the rat liver enzymes that hydrolyze benzoyl-arginine-2-naphthylamide. Biochim Biophys Acta. 1975 Aug 26;397(2):437–442. doi: 10.1016/0005-2744(75)90133-3. [DOI] [PubMed] [Google Scholar]
- Dayton W. R., Goll D. E., Zeece M. G., Robson R. M., Reville W. J. A Ca2+-activated protease possibly involved in myofibrillar protein turnover. Purification from porcine muscle. Biochemistry. 1976 May 18;15(10):2150–2158. doi: 10.1021/bi00655a019. [DOI] [PubMed] [Google Scholar]
- Dayton W. R., Reville W. J., Goll D. E., Stromer M. H. A Ca2+-activated protease possibly involved in myofibrillar protein turnover. Partial characterization of the purified enzyme. Biochemistry. 1976 May 18;15(10):2159–2167. doi: 10.1021/bi00655a020. [DOI] [PubMed] [Google Scholar]
- Etherington D. J. The purification of bovine cathepsin B1 and its mode of action on bovine collagens. Biochem J. 1974 Mar;137(3):547–557. doi: 10.1042/bj1370547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson J. B., Andrews J. R., Voynick I. M., Fruton J. S. The specificity of cathepsin D. J Biol Chem. 1973 Oct 10;248(19):6701–6708. [PubMed] [Google Scholar]
- Franklin S. G., Metrione R. M. Chromatographic evidence for the existence of multiple forms of cathepsin B1. Biochem J. 1972 Mar;127(1):207–213. doi: 10.1042/bj1270207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman I., Laufer A., Davies A. M. Studies on lysosomes in rat heart cell cultures. II. The effect of exogenous lysosomes. Experientia. 1969 Oct 15;25(10):1092–1093. doi: 10.1007/BF01901451. [DOI] [PubMed] [Google Scholar]
- Goldspink D. F., Harris J. B., Park D. C., Pennington R. J. Quantitative enzyme studies in extensor digitorum longus and soleus muscles of rats. Enzymol Biol Clin (Basel) 1970;11(6):481–490. doi: 10.1159/000458390. [DOI] [PubMed] [Google Scholar]
- Goldspink G. Changes in striated muscle fibres during contraction and growth with particular reference to myofibril splitting. J Cell Sci. 1971 Jul;9(1):123–137. doi: 10.1242/jcs.9.1.123. [DOI] [PubMed] [Google Scholar]
- Hoffstein S., Gennaro D. E., Weissmann G., Hirsch J., Streuli F., Fox A. C. Cytochemical localization of lysosomal enzyme activity in normal and ischemic dog myocardium. Am J Pathol. 1975 May;79(2):193–206. [PMC free article] [PubMed] [Google Scholar]
- Huston R. B., Krebs E. G. Activation of skeletal muscle phosphorylase kinase by Ca2+. II. Identification of the kinase activating factor as a proteolytic enzyme. Biochemistry. 1968 Jun;7(6):2116–2122. doi: 10.1021/bi00846a014. [DOI] [PubMed] [Google Scholar]
- Jensen M. S., Bainton D. F. Temporal changes in pH within the phagocytic vacuole of the polymorphonuclear neutrophilic leukocyte. J Cell Biol. 1973 Feb;56(2):379–388. doi: 10.1083/jcb.56.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järvinen M., Hopsu-Havu V. K. alpha-N-benzoylarginine-2-naphthylamide hydrolase (cathepsin B1 ?) from rat skin. II. Purification of the enzyme and demonstration of two inhibitors in the skin. Acta Chem Scand B. 1975;29(7):772–780. doi: 10.3891/acta.chem.scand.29b-0772. [DOI] [PubMed] [Google Scholar]
- Kar N. C., Pearson C. M. A calcium-activated neutral protease in normal and dystrophic human muscle. Clin Chim Acta. 1976 Dec 1;73(2):293–297. doi: 10.1016/0009-8981(76)90175-3. [DOI] [PubMed] [Google Scholar]
- Katunuma N., Kominami E., Kobayashi K., Banno Y., Suzuki K. Studies on new intracellular proteases in various organs of rat. 1. Purification and comparison of their properties. Eur J Biochem. 1975 Mar 3;52(1):37–50. doi: 10.1111/j.1432-1033.1975.tb03970.x. [DOI] [PubMed] [Google Scholar]
- Kirschke H., Langner J., Wiederanders B., Ansorge S., Bohley P., Broghammer U. Intrazellulärer Proteinabbau. VII. Kathepsin L und H: Zwei neue Proteinasen aus Rattenleberlysosomen. Acta Biol Med Ger. 1976;35(3-4):285–299. [PubMed] [Google Scholar]
- Kohn R. R. A proteolytic system involving myofibrils and a soluble factor from normal and atrophying muscle. Lab Invest. 1969 Feb;20(2):202–206. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Mayer M., Khassis S., Shafrir E. Determination of trypsin by its accelerating effect on the onset of trypsinogen activation. Anal Biochem. 1974 Mar;58(1):25–29. doi: 10.1016/0003-2697(74)90436-9. [DOI] [PubMed] [Google Scholar]
- McGowan E. B., Shafiq S. A., Stracher A. Delayed degeneration of dystrophic and normal muscle cell cultures treated with pepstatin, leupeptin, and antipain. Exp Neurol. 1976 Mar;50(3):649–657. doi: 10.1016/0014-4886(76)90034-0. [DOI] [PubMed] [Google Scholar]
- Offer G., Moos C., Starr R. A new protein of the thick filaments of vertebrate skeletal myofibrils. Extractions, purification and characterization. J Mol Biol. 1973 Mar 15;74(4):653–676. doi: 10.1016/0022-2836(73)90055-7. [DOI] [PubMed] [Google Scholar]
- Ogino K., Nakashima K. Purification of rabbit liver cathepsin B1. J Biochem. 1974 Apr;75(4):723–730. doi: 10.1093/oxfordjournals.jbchem.a130445. [DOI] [PubMed] [Google Scholar]
- Otto K., Riesenkönig H. Improved purification of cathepsin B1 and cathepsin B2. Biochim Biophys Acta. 1975 Feb 27;379(2):462–475. doi: 10.1016/0005-2795(75)90153-1. [DOI] [PubMed] [Google Scholar]
- Park D. C., Pennington R. J. Proteinase activity in muscle particles. Enzymol Biol Clin (Basel) 1967;8(2):149–160. doi: 10.1159/000458186. [DOI] [PubMed] [Google Scholar]
- REISFELD R. A., LEWIS U. J., WILLIAMS D. E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- Sapolsky A. I., Woessner J. F., Jr Multiple forms of cathepsin D from bovine uterus. J Biol Chem. 1972 Apr 10;247(7):2069–2076. [PubMed] [Google Scholar]
- Seraydarian K., Briskey E. J., Mommaerts W. F. The modification of actomyosin by alpha- actinin. I. A survey of experimental conditions. Biochim Biophys Acta. 1967 Apr 11;133(3):399–411. doi: 10.1016/0005-2795(67)90544-2. [DOI] [PubMed] [Google Scholar]
- Smith R., Turk V. Cathepsin D: rapid isolation by affinity chromatography on haemoglobin-agarose resin. Eur J Biochem. 1974 Oct 1;48(1):245–254. doi: 10.1111/j.1432-1033.1974.tb03762.x. [DOI] [PubMed] [Google Scholar]
- Suominen J., Hopsu-Havu V. K. Cathepsin B' in the thyroid gland. Acta Chem Scand. 1971;25(7):2531–2540. doi: 10.3891/acta.chem.scand.25-2531. [DOI] [PubMed] [Google Scholar]
- Swanson A. A., Martin B. J., Spicer S. S. Human placental cathepsin B1. Isolation and some physical properties. Biochem J. 1974 Feb;137(2):223–228. doi: 10.1042/bj1370223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towatari T., Tanaka K., Yoshikawa D., Katunuma N. Separation of a new protease from cathepsin B1 of rat liver lysosomes. FEBS Lett. 1976 Sep 1;67(3):284–288. doi: 10.1016/0014-5793(76)80548-0. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]