Abstract
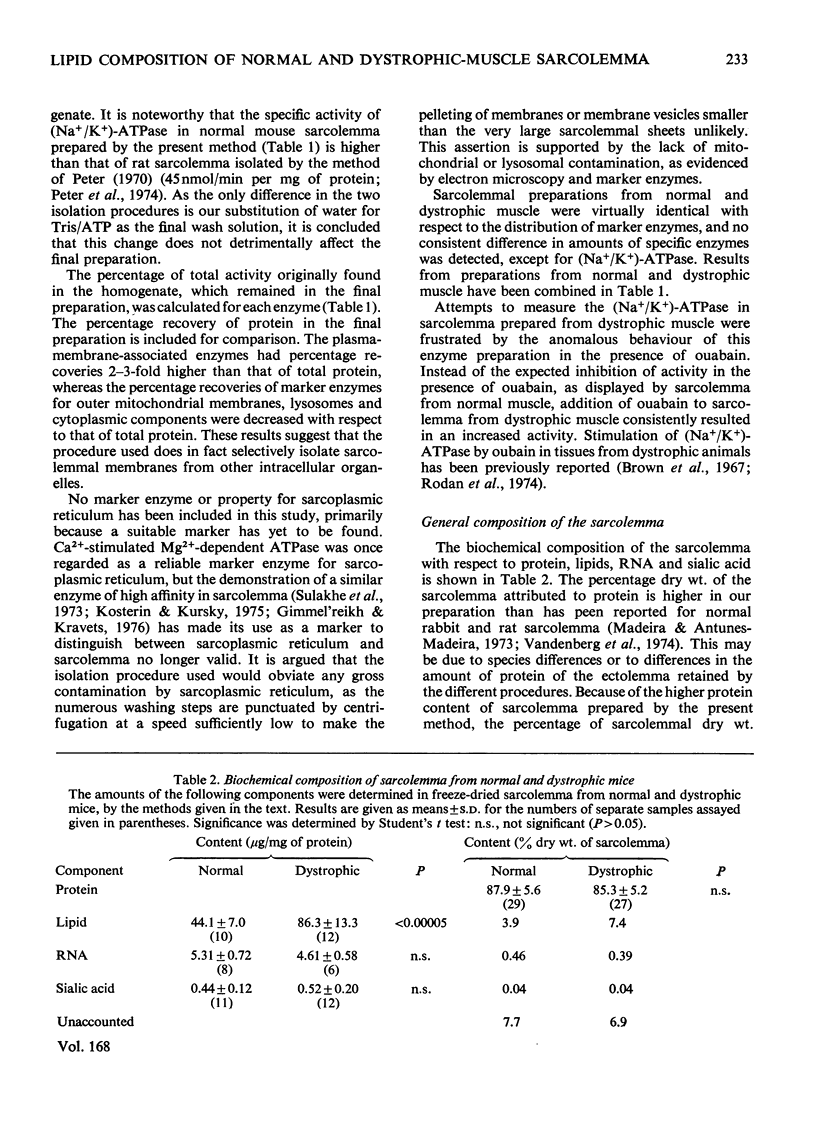
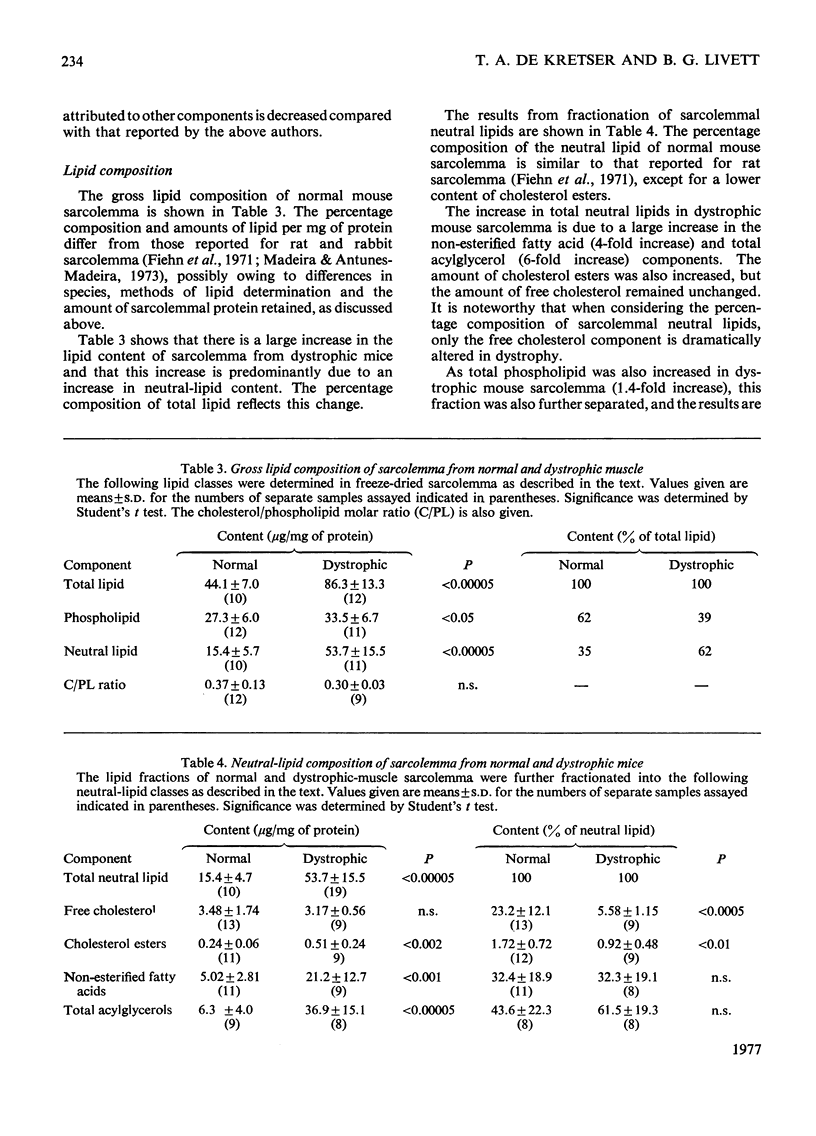
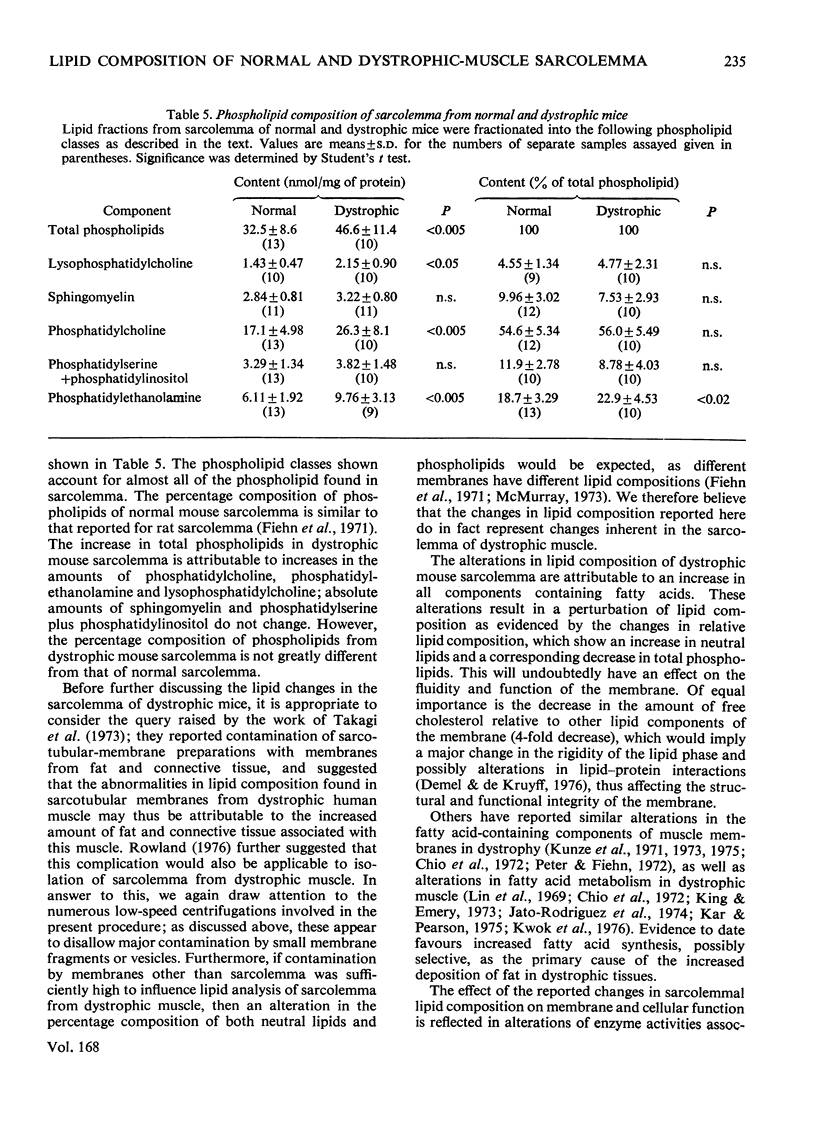
1. Mouse skeletal-muscle sarcolemma was isolated, and the preparations obtained from normal mouse muscle and from muscle of mice with hereditary muscular dystrophy were characterized with respect to appearance under the optical and electron microscopes, distribution of marker enzymes, histochemical properties and biochemical composition. 2. The sarcolemmal membranes from normal and dystrophic muscle were subjected to detailed lipied analysis. Total lipid content was shown to increase in sarcolemma from dystrophic mice as a result of a large increase in neutral lipid and a smaller increase in total phospholipids. Further analysis of the neutral-lipid fraction showed that total acylglycerols increased 6-fold, non-esterified fatty acid 4-fold and cholesterol esters 2-fold, whereas the amount of free cholesterol remained unchanged in sarcolemma from dystrophic muscle. Significant increases were found in lysophosphatidylcholine, phosphatidylcholine and phosphatidylethanolamine in dystrophic-muscle sarcolemma; however, the relative composition of the phospholipid fraction remained essentially the same as in the normal case. 3. The overall result of alterations in lipid composition of the sarcolemma in mouse muscular dystrophy was an increase in neutral lipid compared with total phospholipid, and a 4-fold decrease in the relative amount of free cholesterol in the membrane. The possible impact of these changes on membrane function is discussed.
Full text
PDF




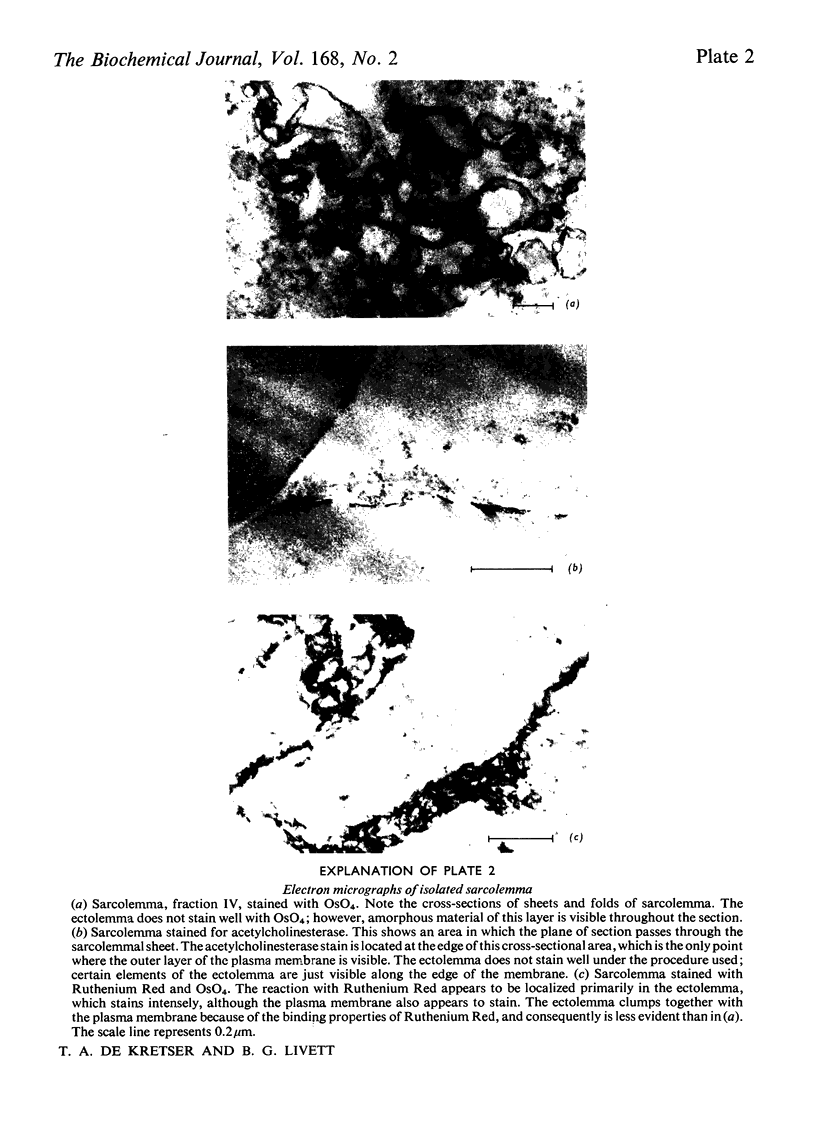
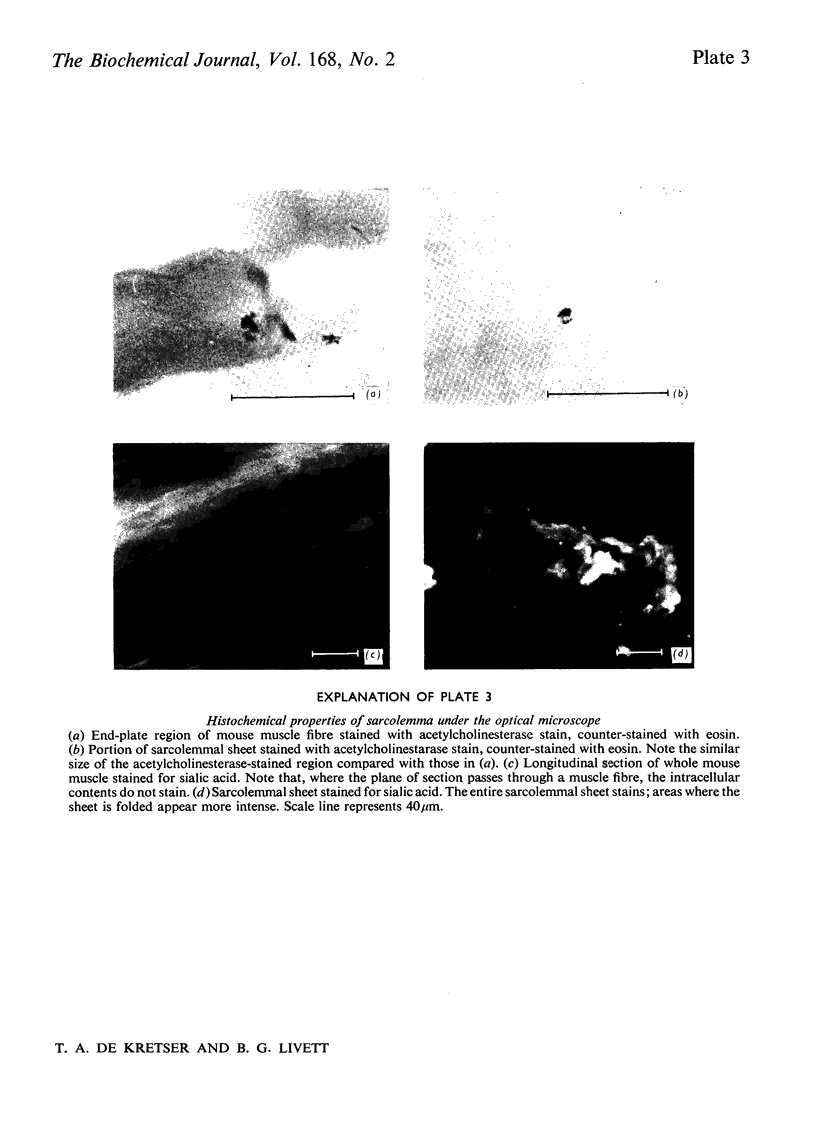


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beringer T., Koenig E. The sarcolemmal membrane: evacuation of short myofiber bundles. Exp Neurol. 1975 Jan;46(1):20–31. doi: 10.1016/0014-4886(75)90028-x. [DOI] [PubMed] [Google Scholar]
- Bondjers G., Björkerud S. Fluorometric determination of cholesterol and cholesteryl ester in tissue on the nanogram level. Anal Biochem. 1971 Aug;42(2):363–371. doi: 10.1016/0003-2697(71)90049-2. [DOI] [PubMed] [Google Scholar]
- Brown H. D., Chattopadhyay S. K., Patel A. B. Erythrocyte abnormality in human myopathy. Science. 1967 Sep 29;157(3796):1577–1578. doi: 10.1126/science.157.3796.1577. [DOI] [PubMed] [Google Scholar]
- Chio L. F., Peterson D. W., Kratzer F. H. Lipid composition and synthesis in the muscles of normal and dystrophic chickens. Can J Biochem. 1972 Dec;50(12):1267–1272. doi: 10.1139/o72-172. [DOI] [PubMed] [Google Scholar]
- Cotman C. W., Matthews D. A. Synaptic plasma membranes from rat brain synaptosomes: isolation and partial characterization. Biochim Biophys Acta. 1971 Dec 3;249(2):380–394. doi: 10.1016/0005-2736(71)90117-9. [DOI] [PubMed] [Google Scholar]
- DePierre J. W., Karnovsky M. L. Plasma membranes of mammalian cells: a review of methods for their characterization and isolation. J Cell Biol. 1973 Feb;56(2):275–303. doi: 10.1083/jcb.56.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demel R. A., De Kruyff B. The function of sterols in membranes. Biochim Biophys Acta. 1976 Oct 26;457(2):109–132. doi: 10.1016/0304-4157(76)90008-3. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L., COURTNEY K. D., ANDRES V., Jr, FEATHER-STONE R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961 Jul;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- EMMELOT P., BOS C. J., BENEDETTI E. L., RUEMKE P. STUDIES ON PLASMA MEMBRANES. I. CHEMICAL COMPOSITION AND ENZYME CONTENT OF PLASMA MEMBRANES ISOLATED FROM RAT LIVER. Biochim Biophys Acta. 1964 Jul 15;90:126–145. doi: 10.1016/0304-4165(64)90125-4. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fiehn W., Peter J. B., Mead J. F., Gan-Elepano M. Lipids and fatty acids of sarcolemma, sarcoplasmic reticulum, and mitochondria from rat skeletal muscle. J Biol Chem. 1971 Sep 25;246(18):5617–5620. [PubMed] [Google Scholar]
- Frings C. S., Dunn R. T. A colorimetric method for determination of total serum lipids based on the sulfo-phospho-vanillin reaction. Am J Clin Pathol. 1970 Jan;53(1):89–91. doi: 10.1093/ajcp/53.1.89. [DOI] [PubMed] [Google Scholar]
- Hammond K. S., Papermaster D. S. Fluorometric assay of sialic acid in the picomole range: a modification of the thiobarbituric acid assay. Anal Biochem. 1976 Aug;74(2):292–297. doi: 10.1016/0003-2697(76)90210-4. [DOI] [PubMed] [Google Scholar]
- Hess H. H., Derr J. E. Assay of inorganic and organic phosphorus in the 0.1-5 nanomole range. Anal Biochem. 1975 Feb;63(2):607–613. doi: 10.1016/0003-2697(75)90388-7. [DOI] [PubMed] [Google Scholar]
- Jato-Rodriguez J. J., Hudson A. J., Strickland K. P. Triglyceride metabolism in skeletal muscle from normal and dystrophic mice. Biochim Biophys Acta. 1974 Apr 26;348(1):1–13. doi: 10.1016/0005-2760(74)90087-3. [DOI] [PubMed] [Google Scholar]
- KARNOVSKY M. J., ROOTS L. A "DIRECT-COLORING" THIOCHOLINE METHOD FOR CHOLINESTERASES. J Histochem Cytochem. 1964 Mar;12:219–221. doi: 10.1177/12.3.219. [DOI] [PubMed] [Google Scholar]
- Kar N. C., Pearson C. M. Lipase activity in normal and dystrophic human muscle. Biochem Med. 1975 Sep;14(1):135–137. doi: 10.1016/0006-2944(75)90028-9. [DOI] [PubMed] [Google Scholar]
- Kidwai A. M., Radcliffe M. A., Lee E. Y., Daniel E. E. Isolation and properties of skeletal muscle plasma membrane. Biochim Biophys Acta. 1973 Mar 29;298(3):593–607. doi: 10.1016/0005-2736(73)90076-x. [DOI] [PubMed] [Google Scholar]
- King B., Emery A. E. Leucocyte fatty acid oxidation in hereditary neuromuscular disorders. A preliminary report. J Neurol Sci. 1973 Nov;20(3):297–302. doi: 10.1016/0022-510x(73)90191-3. [DOI] [PubMed] [Google Scholar]
- Kosterin S. O., Kursky M. D., Rybalchenko V. K. [Heterogeneous and kinetic analysis of certain properties of Ca2+-ATPase in sarcolemma of rabbit skeletal muscles]. Ukr Biokhim Zh. 1975 Nov-Dec;47(6):708–713. [PubMed] [Google Scholar]
- Kunze D., Olthoff D., Hübschmann K. The lipid content of musculature and of tissue preparations with enriched plasma membranes in paramyotonia congenita. Experientia. 1971 Jul;27(7):775–776. doi: 10.1007/BF02136858. [DOI] [PubMed] [Google Scholar]
- Kunze D., Reichmann G., Egger E., Leuschner G., Eckhardt H. Erythrozytenlipide bei Progressiver Muskeldystrophie. Clin Chim Acta. 1973 Feb 12;43(3):333–341. doi: 10.1016/0009-8981(73)90471-3. [DOI] [PubMed] [Google Scholar]
- Kunze D., Reichmann G., Egger E., Olthoff D., Döhler K. Fatty acid pattern of lipids in normal and dystrophic human muscle. Eur J Clin Invest. 1975 Nov 21;5(6):471–475. doi: 10.1111/j.1365-2362.1975.tb00479.x. [DOI] [PubMed] [Google Scholar]
- Kwok C. T., Kuffer A. D., Tang B. Y., Austin L. Phospholipid metabolism in murine muscular dystrophy. Exp Neurol. 1976 Feb;50(2):362–375. doi: 10.1016/0014-4886(76)90011-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lin C. H., Hudson A. J., Strickland K. P. Adenyl cyclase and cyclic nucleotide phosphodiesterase activities in murine muscular dystrophy. Enzyme. 1976;21(1):85–95. doi: 10.1159/000458844. [DOI] [PubMed] [Google Scholar]
- Lin C. H., Hudson A. J., Strickland K. P. Fatty acid metabolism in dystrophic muscle in vitro. Life Sci. 1969 Jan 15;8(2):21–26. doi: 10.1016/0024-3205(69)90112-x. [DOI] [PubMed] [Google Scholar]
- Madeira V. M., Antunes-Madeira M. C. Chemical composition of sarcolemma isolated from rabbit skeletal muscle. Biochim Biophys Acta. 1973 Mar 16;298(2):230–238. doi: 10.1016/0005-2736(73)90353-2. [DOI] [PubMed] [Google Scholar]
- Marlarkey W. B., Mendall J. R. Failure of serotonin inhibitor to effect nocturnal GH and prolactin secretion in patients with Duchenne muscular dystrophy. J Clin Endocrinol Metab. 1976 Oct;43(4):889–892. doi: 10.1210/jcem-43-4-889. [DOI] [PubMed] [Google Scholar]
- Mawatari S., Schonberg M., Olarte M. Biochemical abnormalities of erythrocyte membranes in Duchenne dystrophy. Adenosine triphosphatase and adenyl cyclase. Arch Neurol. 1976 Jul;33(7):489–493. doi: 10.1001/archneur.1976.00500070031006. [DOI] [PubMed] [Google Scholar]
- Peter J. B. A (Na+ K+) ATPase of sarcolemma from skeletal muscle. Biochem Biophys Res Commun. 1970 Sep 30;40(6):1362–1367. doi: 10.1016/0006-291x(70)90016-1. [DOI] [PubMed] [Google Scholar]
- Rodan S. B., Hintz R. L., Sha'afi R. I., Rodan G. A. The activity of membrane bound enzymes in muscular dystrophic chicks. Nature. 1974 Dec 13;252(5484):589–591. doi: 10.1038/252589a0. [DOI] [PubMed] [Google Scholar]
- Rosenthal S. L., Edelman P. M., Schwartz I. L. A method for the preparation of skeletal muscle sarcolemma. Biochim Biophys Acta. 1965 Nov 29;109(2):512–517. doi: 10.1016/0926-6585(65)90176-7. [DOI] [PubMed] [Google Scholar]
- Rowland L. P. Pathogenesis of muscular dystrophies. Arch Neurol. 1976 May;33(5):315–321. doi: 10.1001/archneur.1976.00500050001001. [DOI] [PubMed] [Google Scholar]
- Smith S. W. A new salting-out technique for colorimetric free fatty acid assays. Anal Biochem. 1975 Aug;67(2):531–539. doi: 10.1016/0003-2697(75)90329-2. [DOI] [PubMed] [Google Scholar]
- Somogyi P., Chubb I. W., Smith A. D. A possible structural basis for the extracellular release of acetylcholinesterase. Proc R Soc Lond B Biol Sci. 1975 Nov 18;191(1103):271–283. doi: 10.1098/rspb.1975.0128. [DOI] [PubMed] [Google Scholar]
- Sulakhe P. V., Drummond G. I., Ng D. C. Calcium binding by skeletal muscle sarcolemma. J Biol Chem. 1973 Jun 25;248(12):4150–4157. [PubMed] [Google Scholar]
- TSANEV R., MARKOV G. G. Substances interfering with spectrophotometric estimation of nucleic acids and their elimination by the two-wavelength method. Biochim Biophys Acta. 1960 Aug 26;42:442–452. doi: 10.1016/0006-3002(60)90822-2. [DOI] [PubMed] [Google Scholar]
- Takagi A., Schotland D. L., Rowland L. P. Sarcoplasmic reticulum in Duchenne muscular dystrophy. Arch Neurol. 1973 Jun;28(6):380–384. doi: 10.1001/archneur.1973.00490240040006. [DOI] [PubMed] [Google Scholar]
- Takamori M. Contractility and supersensitivity to adrenaline in dystrophic muscle. J Neurol Neurosurg Psychiatry. 1975 May;38(5):483–492. doi: 10.1136/jnnp.38.5.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenburgh H. H., Sheff M. F., Zacks S. I. Chemical composition of isolated rat skeletal sarcolemma. J Membr Biol. 1974;17(1):1–12. doi: 10.1007/BF01870168. [DOI] [PubMed] [Google Scholar]
- Wallach D. F., Lin P. S. A critical evaluation of plasma membrane fractionation. Biochim Biophys Acta. 1973 Nov;300(3):211–254. doi: 10.1016/0304-4157(73)90005-1. [DOI] [PubMed] [Google Scholar]
- Weber P., Harrison F. W., Hof L. The histochemical application of dansylhydrazine as a fluorescent labeling reagent for sialic acid in cellular glycoconjugates. Histochemistry. 1975 Dec 19;45(4):271–277. doi: 10.1007/BF00492629. [DOI] [PubMed] [Google Scholar]
- Zacks S. I., Sheff M. F., Saito A. Structure and staining characteristics of myofiber external lamina. J Histochem Cytochem. 1973 Aug;21(8):703–714. doi: 10.1177/21.8.703. [DOI] [PubMed] [Google Scholar]