Abstract
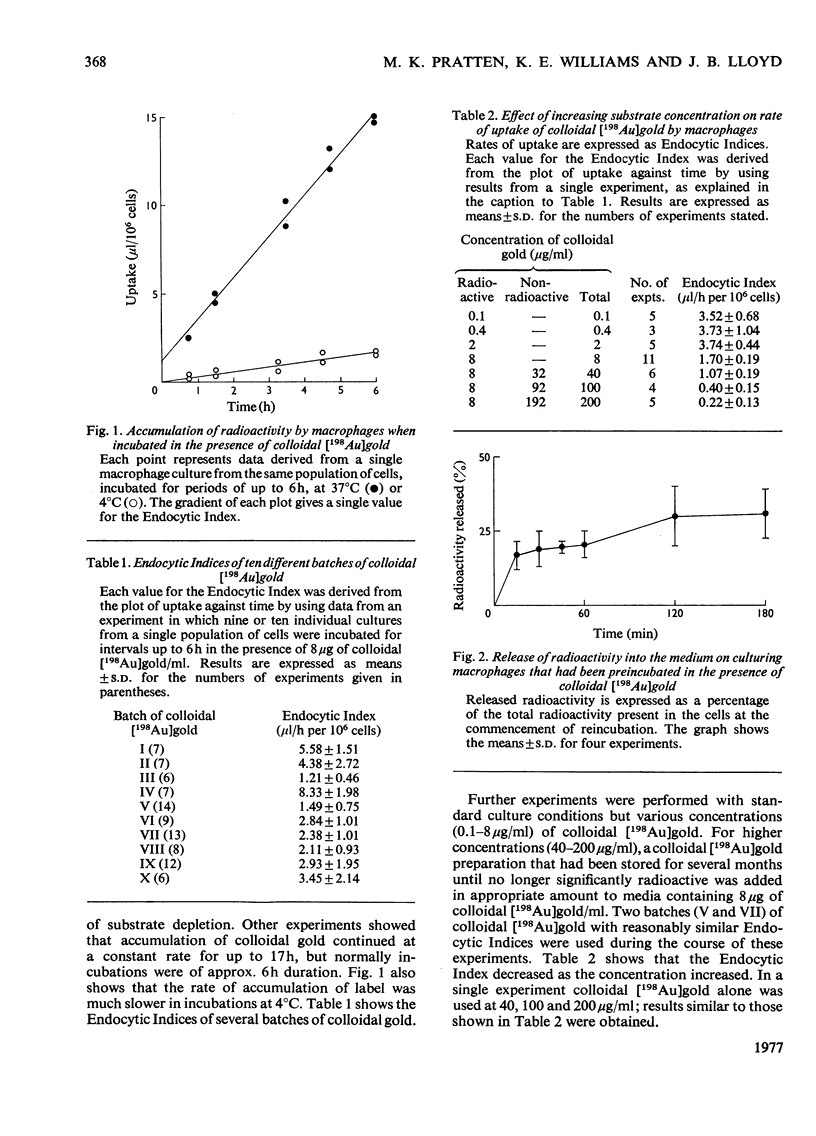
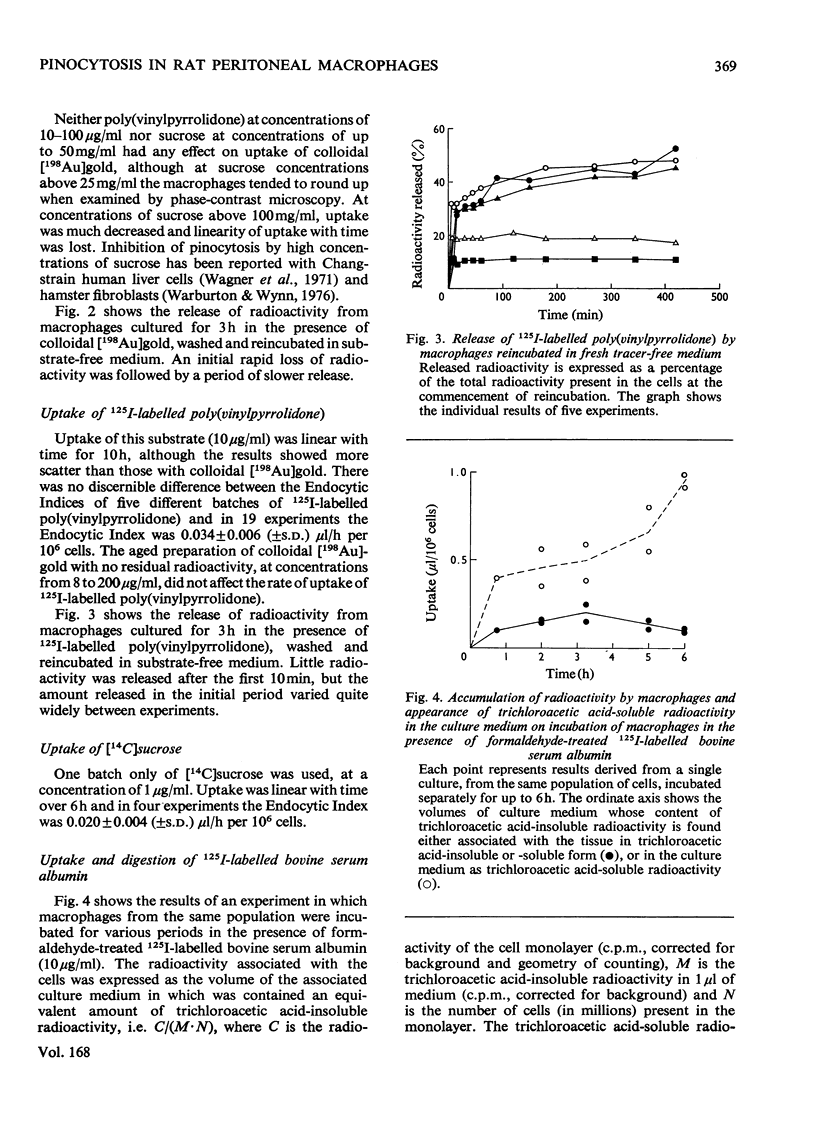
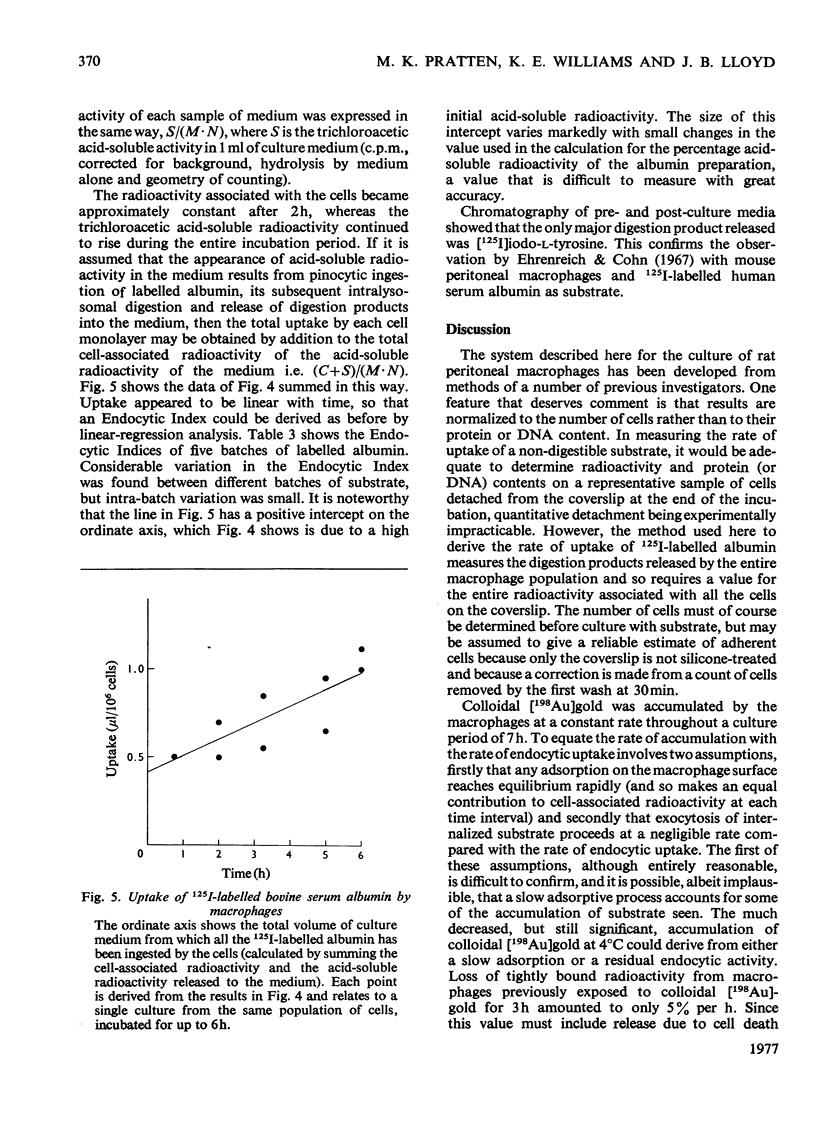
A method for the culture of rat peritoneal macrophages in vitro is described, in which pinocytic uptake of colloidal [198 Au]gold, 125I--labelled poly(vinylpyrrolidone) and [14C]sucrose proceeds at contant and fairly reproducible rates for several hours. The rat of uptake of colloidal [198 Au]gold, which wxhibited some inter-batch variation, was approx. 100 times that of the other two substrates. Colloidal gold did not affect the rate of uptake of 125I-labelled poly(vinylpyrrolidone) and therefore its own high rate of uptake could not be attributed to a stimulation of the formation of pinocytic vesicles. It conclude that uptake of collodial gold is highly dependent on adsorption on binding sites on the plasma membrane. Uptake of formaldehyde-treated 125I-labelled bovine serum albumin was followed by the release of [125I]iodo-L-tyrosine into the culture medium and took place at a rate intermediate between those of collodial [198Au]gold and the other two non-digestible substrates, 125I-labelled poly(vinylpyrrolidone) and [14C]sucrose.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohn Z. A., Parks E. The regulation of pinocytosis in mouse macrophages. 3. The induction of vesicle formation by nucleosides and nucleotides. J Exp Med. 1967 Mar 1;125(3):457–466. doi: 10.1084/jem.125.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn Z. A., Parks E. The regulation of pinocytosis in mouse macrophages. II. Factors inducing vesicle formation. J Exp Med. 1967 Feb 1;125(2):213–232. doi: 10.1084/jem.125.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn Z. A., Parks E. The regulation of pinocytosis in mouse macrophages. IV. The immunological induction of pinocytic vesicles, secondary lysosomes, and hydrolytic enzymes. J Exp Med. 1967 Jun 1;125(6):1091–1104. doi: 10.1084/jem.125.6.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn Z. A. The regulation of pinocytosis in mouse macrophages. I. Metabolic requirements as defined by the use of inhibitors. J Exp Med. 1966 Oct 1;124(4):557–571. doi: 10.1084/jem.124.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P., Allison A. C., Haswell A. D. Selective release of lysosomal hydrolases from phagocytic cells by cytochalasin B. Biochem J. 1973 May;134(1):33–41. doi: 10.1042/bj1340033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P., Allison A. C., Haswell A. D. The quantitative estimation of pinocytosis using radioactive colloidal gold. Biochem Biophys Res Commun. 1973 May 15;52(2):627–634. doi: 10.1016/0006-291x(73)90759-6. [DOI] [PubMed] [Google Scholar]
- Dingle J. T., Poole A. R., Lazarus G. S., Barrett A. J. Immunoinhibition of intracellular protein digestion in macrophages. J Exp Med. 1973 May 1;137(5):1124–1141. doi: 10.1084/jem.137.5.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenreich B. A., Cohn Z. A. Fate of hemoglobin pincytosed by macrophages in vitro. J Cell Biol. 1968 Jul;38(1):244–248. doi: 10.1083/jcb.38.1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenreich B. A., Cohn Z. A. The fate of peptides pinocytosed by macrophages in vitro. J Exp Med. 1969 Jan 1;129(1):227–245. doi: 10.1084/jem.129.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenreich B. A., Cohn Z. A. The uptake and digestion of iodinated human serum albumin by macrophages in vitro. J Exp Med. 1967 Nov 1;126(5):941–958. doi: 10.1084/jem.126.5.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOSSELIN R. E. The uptake of radiocolloids by macrophages in vitro; a kinetic analysis with radioactive colloidal gold. J Gen Physiol. 1956 May 20;39(5):625–649. doi: 10.1085/jgp.39.5.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselin R. E. Kinetics of pinocytosis. Fed Proc. 1967 Jul-Aug;26(4):987–993. [PubMed] [Google Scholar]
- Moore A. T., Williams K. E., Lloyd J. B. The effect of chemical treatments of albumin and orosomucoid on rate of clearance from the rat bloodstream and rate of pinocytic capture of rat yolk sac cultured in vitro. Biochem J. 1977 Jun 15;164(3):607–616. doi: 10.1042/bj1640607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. V., Williams K. E., Lloyd J. B. The pinocytosis of 125I-labelled poly(vinylpyrrolidone), [14C]sucrose and colloidal [198Au]gold by rat yolk sac cultured in vitro. Biochem J. 1977 Nov 15;168(2):239–244. doi: 10.1042/bj1680239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Cohn Z. A. The interaction of soluble horseradish peroxidase with mouse peritoneal macrophages in vitro. J Cell Biol. 1972 Oct;55(1):186–204. doi: 10.1083/jcb.55.1.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R., Rosenberg M., Estensen R. Endocytosis in Chang liver cells. Quantitation by sucrose- 3 H uptake and inhibition by cytochalasin B. J Cell Biol. 1971 Sep;50(3):804–817. doi: 10.1083/jcb.50.3.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton M. J., Wynn C. H. The hyperactivity of hamster fibroblast lysosomal enzymes after endocytosis of sucrose. Biochem Biophys Res Commun. 1976 May 3;70(1):94–100. doi: 10.1016/0006-291x(76)91113-x. [DOI] [PubMed] [Google Scholar]
- Westwood F. R., Longstaff E. Stimulation of cellular ingestion by basic proteins in vitro. Br J Cancer. 1976 Apr;33(4):392–399. doi: 10.1038/bjc.1976.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K. E., Kidston E. M., Beck F., Lloyd J. B. Quantitative studies of pinocytosis. I. Kinetics of uptake of (125I)polyvinylpyrrolidone by rat yolk sac cultured in vitro. J Cell Biol. 1975 Jan;64(1):113–122. doi: 10.1083/jcb.64.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K. E., Kidston E. M., Beck F., Lloyd J. B. Quantitative studies of pinocytosis. II. Kinetics of protein uptake and digestion by rat yolk sac cultured in vitro. J Cell Biol. 1975 Jan;64(1):123–134. doi: 10.1083/jcb.64.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K. E., Lloyd J. B., Davies M., Beck F. Digestion of an exogenous protein by rat yolk-sac cultured in vitro. Biochem J. 1971 Nov;125(1):303–308. doi: 10.1042/bj1250303. [DOI] [PMC free article] [PubMed] [Google Scholar]


