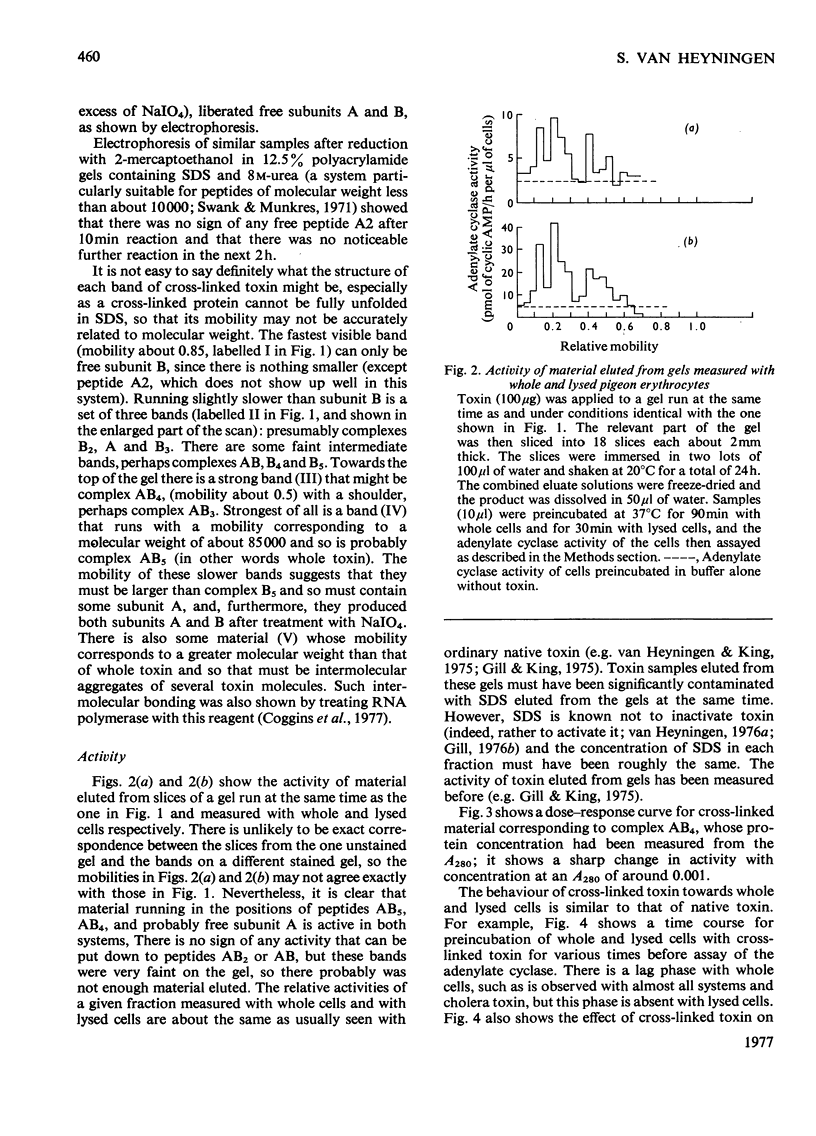
Abstract
Reaction of cholera toxin with NN'-bis(carboximidomethyl)tartaramide dimethyl ester produced several cross-linked species that had subunit B (which binds to the cell surface) and peptides A1 (which activates adenylate cyclase) and A2 all covalently joined together. This cross-linded material had activity with pigeon erythrocytes that was comparable in all respects with that of native toxin. It activated the adenylate cyclase of whole cells, showing a characteristic lag phase, and this activation was increased if the cells had been preincubated with ganglioside GM1, but abolished if the protein had been preincubated with the ganglioside. It activated the enzyme in lysed cells more strongly and without the lag phase. These results show that the toxin is active even when peptide A1 cannot be released from the rest of the molecule.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bitensky M. W., Wheeler M. A., Mehta H., Miki N. Cholera toxin activation of adenylate cyclase in cancer cell membrane fragments. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2572–2576. doi: 10.1073/pnas.72.7.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown B. L., Albano J. D., Ekins R. P., Sgherzi A. M. A simple and sensitive saturation assay method for the measurement of adenosine 3':5'-cyclic monophosphate. Biochem J. 1971 Feb;121(3):561–562. doi: 10.1042/bj1210561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggins J. R., Hooper E. A., Perham R. N. Use of dimethyl suberimidate and novel periodate-cleavable bis(imido esters) to study the quaternary structure of the pyruvate dehydrogenase multienzyme complex of Escherichia coli. Biochemistry. 1976 Jun 15;15(12):2527–2533. doi: 10.1021/bi00657a006. [DOI] [PubMed] [Google Scholar]
- Coggins J. R., Lumsden J., Malcolm A. D. A study of the quaternary structure of Escherichia coli RNA polymerase using bis(imido esters). Biochemistry. 1977 Mar 22;16(6):1111–1116. doi: 10.1021/bi00625a013. [DOI] [PubMed] [Google Scholar]
- Finkelstein R. A., Boesman M., Neoh S. H., LaRue M. K., Delaney R. Dissociation and recombination of the subunits of the cholera enterotoxin (choleragen). J Immunol. 1974 Jul;113(1):145–150. [PubMed] [Google Scholar]
- Fishman P. H., Brady R. O. Biosynthesis and function of gangliosides. Science. 1976 Nov 26;194(4268):906–915. doi: 10.1126/science.185697. [DOI] [PubMed] [Google Scholar]
- Gill D. M. Involvement of nicotinamide adenine dinucleotide in the action of cholera toxin in vitro. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2064–2068. doi: 10.1073/pnas.72.6.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. M., King C. A. The mechanism of action of cholera toxin in pigeon erythrocyte lysates. J Biol Chem. 1975 Aug 25;250(16):6424–6432. [PubMed] [Google Scholar]
- Gill D. M. Multiple roles of erythrocyte supernatant in the activation of adenylate cyclase by Vibrio cholerae toxin in vitro. J Infect Dis. 1976 Mar;133 (Suppl):55–63. doi: 10.1093/infdis/133.supplement_1.s55. [DOI] [PubMed] [Google Scholar]
- Gill D. M. The arrangement of subunits in cholera toxin. Biochemistry. 1976 Mar 23;15(6):1242–1248. doi: 10.1021/bi00651a011. [DOI] [PubMed] [Google Scholar]
- Heyningen S Van Cholera toxin: interaction of subunits with ganglioside GM1. Science. 1974 Feb 15;183(4125):656–657. doi: 10.1126/science.183.4125.656. [DOI] [PubMed] [Google Scholar]
- Heyningen S. Activation by cholera toxin of adenylate cyclase solubilized from rat liver. Biochem J. 1976 Sep 1;157(3):785–787. doi: 10.1042/bj1570785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosky A., Markel D. E., Peterson J. W., Fitch W. M. Primary structure of cholera toxin beta-chain: a glycoprotein hormone analog? Science. 1977 Jan 21;195(4275):299–301. doi: 10.1126/science.831277. [DOI] [PubMed] [Google Scholar]
- Lai C. Y., Mendez E., Chang D., Wang M. Primary structure of cholera toxin B-subunit. Biochem Biophys Res Commun. 1977 Jan 10;74(1):215–222. doi: 10.1016/0006-291x(77)91396-1. [DOI] [PubMed] [Google Scholar]
- Lospalluto J. J., Finkelstein R. A. Chemical and physical properties of cholera exo-enterotoxin (choleragen) and its spontaneously formed toxoid (choleragenoid). Biochim Biophys Acta. 1972 Jan 26;257(1):158–166. doi: 10.1016/0005-2795(72)90265-6. [DOI] [PubMed] [Google Scholar]
- Lönnroth I., Holmgren J. Protein reagent modification of cholera toxin: characterization of effects on antigenic, receptor-binding and toxic properties. J Gen Microbiol. 1975 Dec;91(2):263–277. doi: 10.1099/00221287-91-2-263. [DOI] [PubMed] [Google Scholar]
- Matuo Y., Wheeler M. A., Bitensky M. W. Small fragments from the A subunit of cholera toxin capable of activating adenylate cyclase. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2654–2658. doi: 10.1073/pnas.73.8.2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J., Manganiello V. C., Vaughan M. Hydrolysis of nicotinamide adenine dinucleotide by choleragen and its A protomer: possible role in the activation of adenylate cyclase. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4424–4427. doi: 10.1073/pnas.73.12.4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J., Osborne J. C., Jr, Fishman P. H., Brewer H. B., Jr, Vaughan M., Brady R. O. Effect of gangliosides and substrate analogues on the hydrolysis of nicotinamide adenine dinucleotide by choleragen. Proc Natl Acad Sci U S A. 1977 Jan;74(1):74–78. doi: 10.1073/pnas.74.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima Y., Napiorkowski P., Schafer D. E., Konigsberg W. H. Primary structure of the B subunit of cholera enterotoxin. FEBS Lett. 1976 Oct 1;68(2):275–278. doi: 10.1016/0014-5793(76)80452-8. [DOI] [PubMed] [Google Scholar]
- Sahyoun N., Cuatrecasas P. Mechanism of activation of adenylate cyclase by cholera toxin. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3438–3442. doi: 10.1073/pnas.72.9.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler J., Wiegandt H. Studies of the subunit structure of choleragen. Eur J Biochem. 1975 Sep 1;57(1):309–316. doi: 10.1111/j.1432-1033.1975.tb02302.x. [DOI] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Van Heyningen S., King C. A. Short communications. Subunit A from cholera toxin is an activator of adenylate cyclase in pigeon erythrocytes. Biochem J. 1975 Jan;146(1):269–271. doi: 10.1042/bj1460269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Heyningen W. E., Carpenter C. C., Pierce N. F., Greenough W. B., 3rd Deactivation of cholera toxin by ganglioside. J Infect Dis. 1971 Oct;124(4):415–418. doi: 10.1093/infdis/124.4.415. [DOI] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]


