Abstract
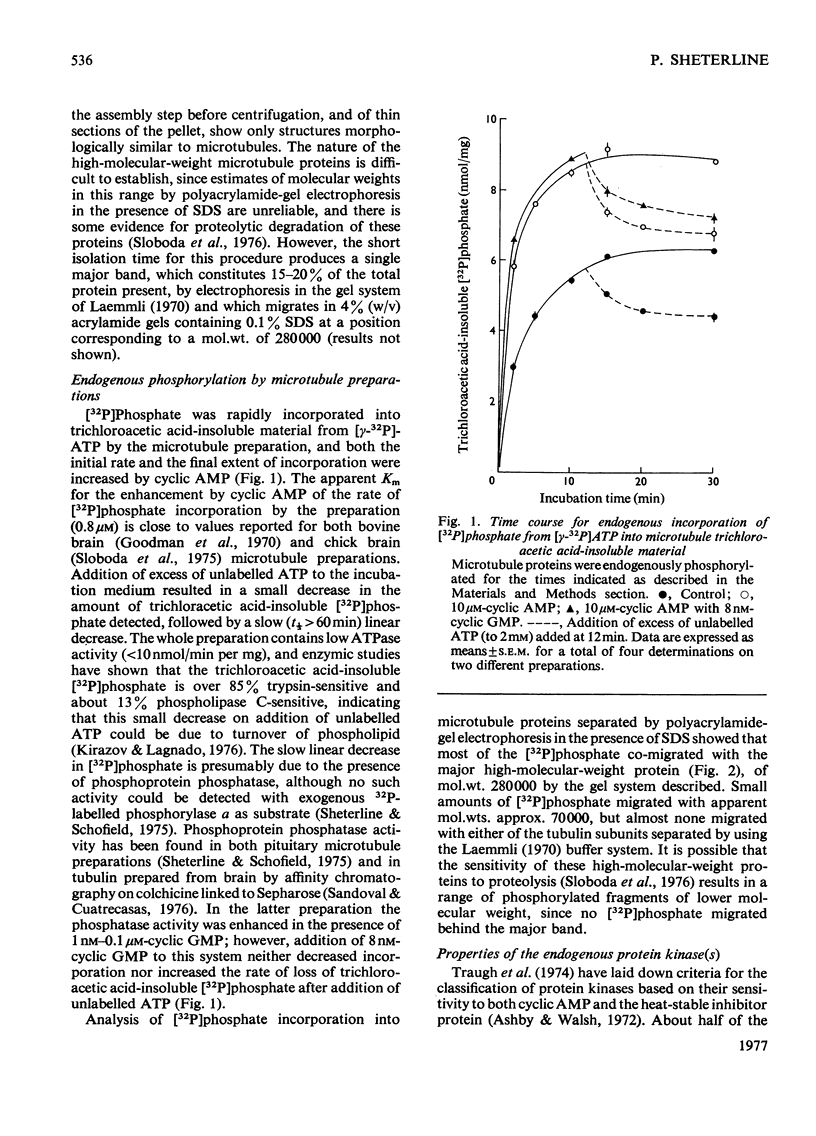
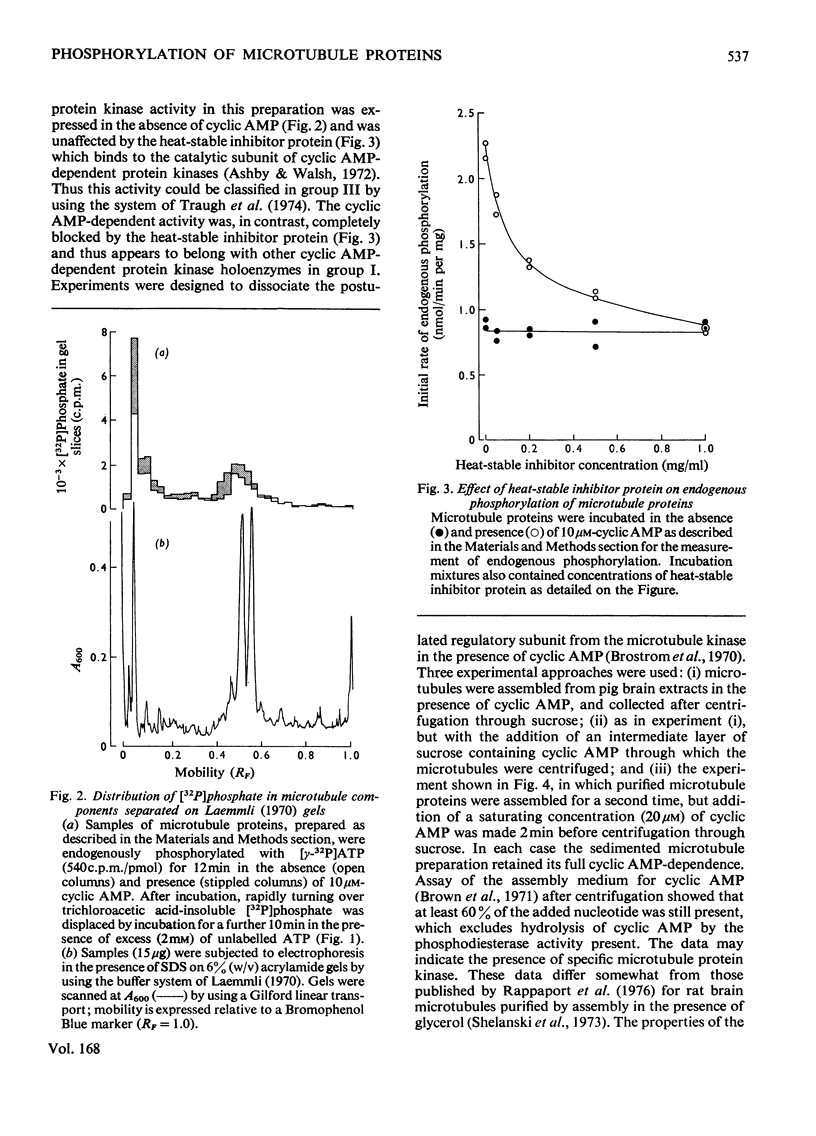
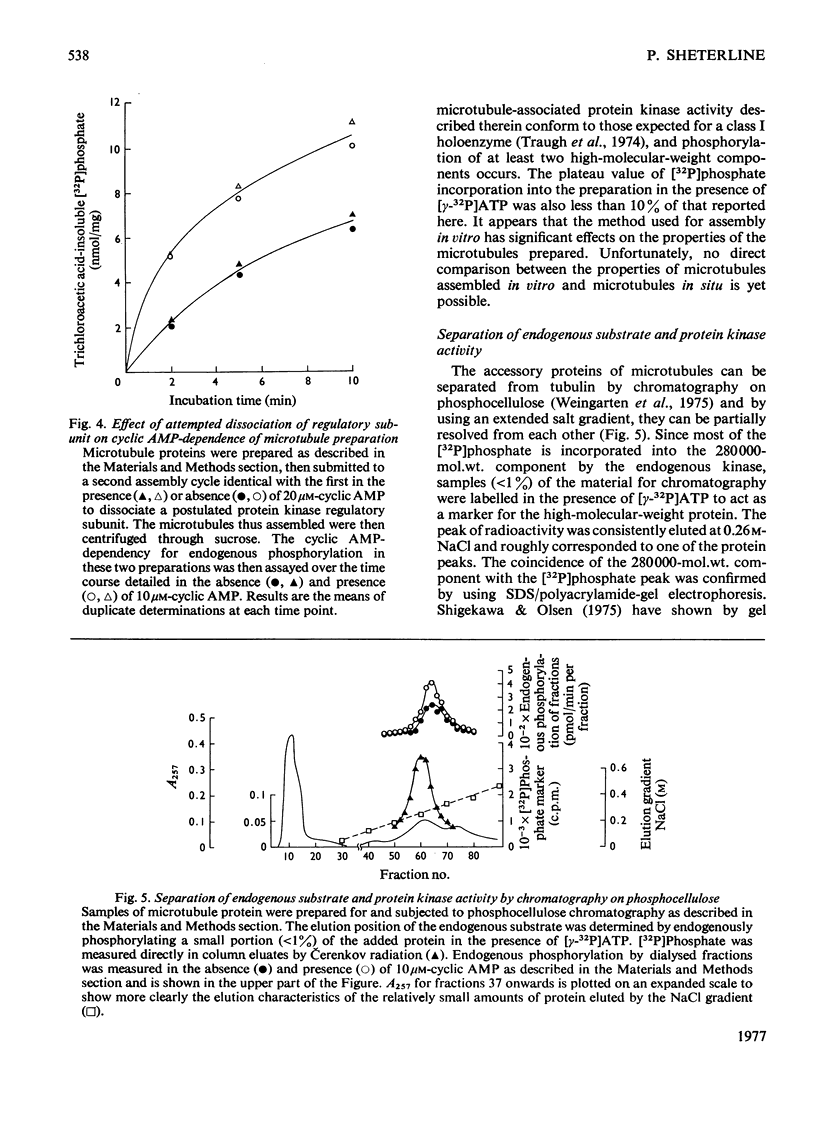
1. A simple purification procedure for microtubule proteins is described, which involves a single assembly step in vitro in the absence of glycerol, followed by centrifugation through sucrose. 2. The preparation contains 80% tubulin (mol.wt. 54000), 15-20% of a 280000-mol.wt. protein and several other minor components of intermediate molecular weight after polyacrylamide-gel electrophoresis in the presence of sodium dodecyl sulphate and 2-mercaptoethanol. 3. In the presence of [gamma-32P]ATP, [32P]phosphate was incorporated into the 280000-mol.wt. component reaching half-maximal incorporation at 1-2 min, but no phosphorylation of tubulin was detected. Cyclic AMP (Km 0.8 micrometer) increased both the initial rate and the extent of incorporation of [32P]phosphate into this component. 4. About half of the endogenous protein kinase activity did not require cyclic AMP and was not inhibited by a heat-stable inhibitor protein from muscle. The remainder of the activity was cyclic AMP-dependent and sensitive to the inhibitor protein. A regulatory subunit was not dissociable from microtubules assembled in vitro in the presence of saturating concentrations of cyclic AMP. 5. The endogenous substrate and the endogenous protein kinase activity could be partially resolved chromatography on phosphocellulose. 6. The data show that cyclic AMP can moduate the activity of an endogenous protein kinase(s) with unusual properties and which phosphorylates a prominent microtubule-associated protein.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amos L. A. Arrangement of high molecular weight associated proteins on purified mammalian brain microtubules. J Cell Biol. 1977 Mar;72(3):642–654. doi: 10.1083/jcb.72.3.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby C. D., Walsh D. A. Characterization of the interaction of a protein inhibitor with adenosine 3',5'-monophosphate-dependent protein kinases. I. Interaction with the catalytic subunit of the protein kinase. J Biol Chem. 1972 Oct 25;247(20):6637–6642. [PubMed] [Google Scholar]
- Beavo J. A., Rogers N. L., Crofford O. B., Hardman J. G., Sutherland E. W., Newman E. V. Effects of xanthine derivatives on lipolysis and on adenosine 3',5'-monophosphate phosphodiesterase activity. Mol Pharmacol. 1970 Nov;6(6):597–603. [PubMed] [Google Scholar]
- Borisy G. G., Marcum J. M., Olmsted J. B., Murphy D. B., Johnson K. A. Purification of tubulin and associated high molecular weight proteins from porcine brain and characterization of microtubule assembly in vitro. Ann N Y Acad Sci. 1975 Jun 30;253:107–132. doi: 10.1111/j.1749-6632.1975.tb19196.x. [DOI] [PubMed] [Google Scholar]
- Borisy G. G., Olmsted J. B., Marcum J. M., Allen C. Microtubule assembly in vitro. Fed Proc. 1974 Feb;33(2):167–174. [PubMed] [Google Scholar]
- Brostrom M. A., Reimann E. M., Walsh D. A., Krebs E. G. A cyclic 3',5'-amp-stimulated protein kinase from cardiac muscle. Adv Enzyme Regul. 1970;8:191–203. doi: 10.1016/0065-2571(70)90017-8. [DOI] [PubMed] [Google Scholar]
- Brown B. L., Albano J. D., Ekins R. P., Sgherzi A. M. A simple and sensitive saturation assay method for the measurement of adenosine 3':5'-cyclic monophosphate. Biochem J. 1971 Feb;121(3):561–562. doi: 10.1042/bj1210561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan J. Biochemical properties of microtubules. Fed Proc. 1974 Feb;33(2):152–157. [PubMed] [Google Scholar]
- Burns R. G., Pollard T. D. A dynein-like protein from brain. FEBS Lett. 1974 Apr 1;40(2):274–280. doi: 10.1016/0014-5793(74)80243-7. [DOI] [PubMed] [Google Scholar]
- Corbin J. D., Reimann E. M. Assay of cyclic AMP-dependent protein kinases. Methods Enzymol. 1974;38:287–290. doi: 10.1016/0076-6879(74)38044-5. [DOI] [PubMed] [Google Scholar]
- Gaskin F., Kramer S. B., Cantor C. R., Adelstein R., Shelanski M. L. A dynein-like protein associated with neurotubules. FEBS Lett. 1974 Apr 1;40(2):281–286. doi: 10.1016/0014-5793(74)80244-9. [DOI] [PubMed] [Google Scholar]
- Goodman D. B., Rasmussen H., DiBella F., Guthrow C. E., Jr Cyclic adenosine 3':5'-monophosphate-stimulated phosphorylation of isolated neurotubule subunits. Proc Natl Acad Sci U S A. 1970 Oct;67(2):652–659. doi: 10.1073/pnas.67.2.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoué S., Sato H. Cell motility by labile association of molecules. The nature of mitotic spindle fibers and their role in chromosome movement. J Gen Physiol. 1967 Jul;50(6 Suppl):259–292. [PMC free article] [PubMed] [Google Scholar]
- Itani A. N., Lagnado J. R. The distribution of colchicine and vinblastine receptors in subcellular fractions from 1--3-day-old chick brain. Biochem Soc Trans. 1976;4(4):732–734. doi: 10.1042/bst0040732. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lagnado J., Tan L. P., Reddington M. The in situ phosphorylation of microtubular protein in brain cortex slices and related studies on the phosphorylation of isolated brain tubulin preparations. Ann N Y Acad Sci. 1975 Jun 30;253:577–597. doi: 10.1111/j.1749-6632.1975.tb19231.x. [DOI] [PubMed] [Google Scholar]
- Mellon M. G., Rebhun L. I. Sulfhydryls and the in vitro polymerization of tubulin. J Cell Biol. 1976 Jul;70(1):226–238. doi: 10.1083/jcb.70.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy D. B., Borisy G. G. Association of high-molecular-weight proteins with microtubules and their role in microtubule assembly in vitro. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2696–2700. doi: 10.1073/pnas.72.7.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy D. B., Tilney L. G. The role of microtubules in the movement of pigment granules in teleost melanophores. J Cell Biol. 1974 Jun;61(3):757–779. doi: 10.1083/jcb.61.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmsted J. B., Borisy G. G. Ionic and nucleotide requirements for microtubule polymerization in vitro. Biochemistry. 1975 Jul;14(13):2996–3005. doi: 10.1021/bi00684a032. [DOI] [PubMed] [Google Scholar]
- Pipeleers D. G., Pipeleers-Marichal M. A., Kipnis D. M. Microtubule assembly and the intracellular transport of secretory granules in pancreatic islets. Science. 1976 Jan 9;191(4222):88–90. doi: 10.1126/science.1108194. [DOI] [PubMed] [Google Scholar]
- Rappaport L., Leterrier J. F., Virion A., Nunez J., Osty J. Phosphorylation of microtubule-associated proteins. Eur J Biochem. 1976 Mar 1;62(3):539–549. doi: 10.1111/j.1432-1033.1976.tb10188.x. [DOI] [PubMed] [Google Scholar]
- Raybin D., Flavin M. Modification of tubulin by tyrosylation in cells and extracts and its effect on assembly in vitro. J Cell Biol. 1977 May;73(2):492–504. doi: 10.1083/jcb.73.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roisen F. J., Murphy R. A., Pichichero M. E., Braden W. G. Cyclic adenosine monophosphate stimulation of axonal elongation. Science. 1972 Jan 7;175(4017):73–74. doi: 10.1126/science.175.4017.73. [DOI] [PubMed] [Google Scholar]
- Sandoval I. V., Cuatrecasas P. Opposing effects of cyclic AMP and cyclic GMP on protein phosphorylation in tubulin preparations. Nature. 1976 Aug 5;262(5568):511–514. doi: 10.1038/262511a0. [DOI] [PubMed] [Google Scholar]
- Shelanski M. L., Gaskin F., Cantor C. R. Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci U S A. 1973 Mar;70(3):765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheterline P. Phosphorylation of microtubule proteins by an endogenous adenosine 3':5'-cyclic monophosphate-dependent protein kinase. Biochem Soc Trans. 1976;4(4):789–791. doi: 10.1042/bst0040789. [DOI] [PubMed] [Google Scholar]
- Sheterline P., Schofield J. G. Endogenous phosphorylation and dephosphorylation of microtubule-associated proteins isolated from bovine anterior pituitary. FEBS Lett. 1975 Aug 15;56(2):297–302. doi: 10.1016/0014-5793(75)81113-6. [DOI] [PubMed] [Google Scholar]
- Sheterline P., Schofield J. G., Mira-Moser F. The effect of secretotogues and 2-methylpentan-2,4-diol on the microtubule-tubulin equilibrium and the release of growth hormone from bovine anterior pituitary slices. Exp Cell Res. 1977 Jan;104(1):127–134. doi: 10.1016/0014-4827(77)90075-1. [DOI] [PubMed] [Google Scholar]
- Shigekawa B. L., Olsen R. W. Resolution of cyclic AMP stimulated protein kinase from polymerization-purified brain microtubules. Biochem Biophys Res Commun. 1975 Mar 17;63(2):455–462. doi: 10.1016/0006-291x(75)90709-3. [DOI] [PubMed] [Google Scholar]
- Sloboda R. D., Rudolph S. A., Rosenbaum J. L., Greengard P. Cyclic AMP-dependent endogenous phosphorylation of a microtubule-associated protein. Proc Natl Acad Sci U S A. 1975 Jan;72(1):177–181. doi: 10.1073/pnas.72.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSUBOI K. K., PRICE T. D. Isolation, detection and measure of microgram quantities of labeled tissue nucleotides. Arch Biochem Biophys. 1959 Mar;81(1):223–237. doi: 10.1016/0003-9861(59)90192-4. [DOI] [PubMed] [Google Scholar]
- Tilney L. G., Bryan J., Bush D. J., Fujiwara K., Mooseker M. S., Murphy D. B., Snyder D. H. Microtubules: evidence for 13 protofilaments. J Cell Biol. 1973 Nov;59(2 Pt 1):267–275. doi: 10.1083/jcb.59.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traugh J. A., Ashby C. D., Walsh D. A. Criteria for the classification of protein kinases. Methods Enzymol. 1974;38:290–299. doi: 10.1016/0076-6879(74)38045-7. [DOI] [PubMed] [Google Scholar]
- Walsh D. A., Perkins J. P., Brosom C. O., Ho E. S., Kreb E. G. Catlysis of the phosphrylaseinase actition reaction. J Biol Chem. 1971 Apr 10;246(7):1968–1976. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weingarten M. D., Lockwood A. H., Hwo S. Y., Kirschner M. W. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975 May;72(5):1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisenberg R. C. Microtubule formation in vitro in solutions containing low calcium concentrations. Science. 1972 Sep 22;177(4054):1104–1105. doi: 10.1126/science.177.4054.1104. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Pastan I. Cyclic amp and cell morphology in cultured fibroblasts. Effects on cell shape, microfilament and microtubule distribution, and orientation to substratum. J Cell Biol. 1975 Oct;67(1):146–159. doi: 10.1083/jcb.67.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahara I., Edelman G. M. Electron microscopic analysis of the modulation of lymphocyte receptor mobility. Exp Cell Res. 1975 Mar 1;91(1):125–142. doi: 10.1016/0014-4827(75)90150-0. [DOI] [PubMed] [Google Scholar]


