Abstract
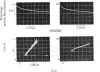
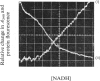
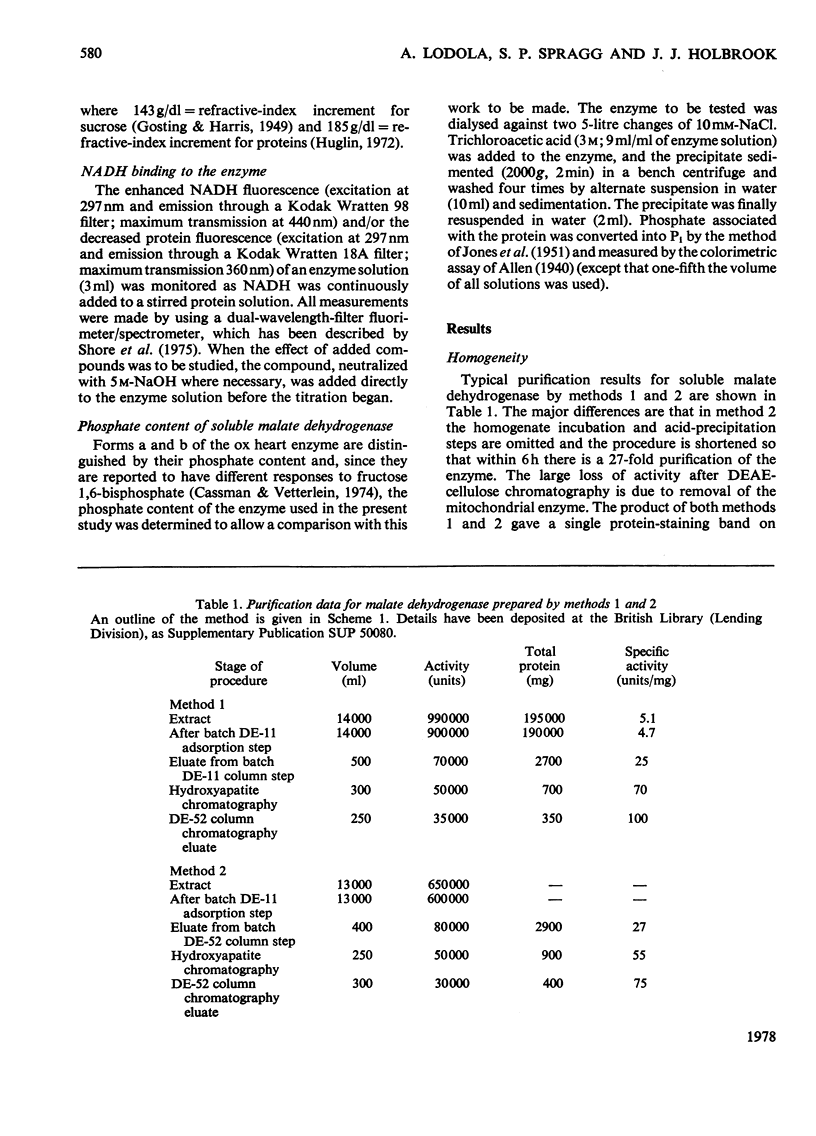
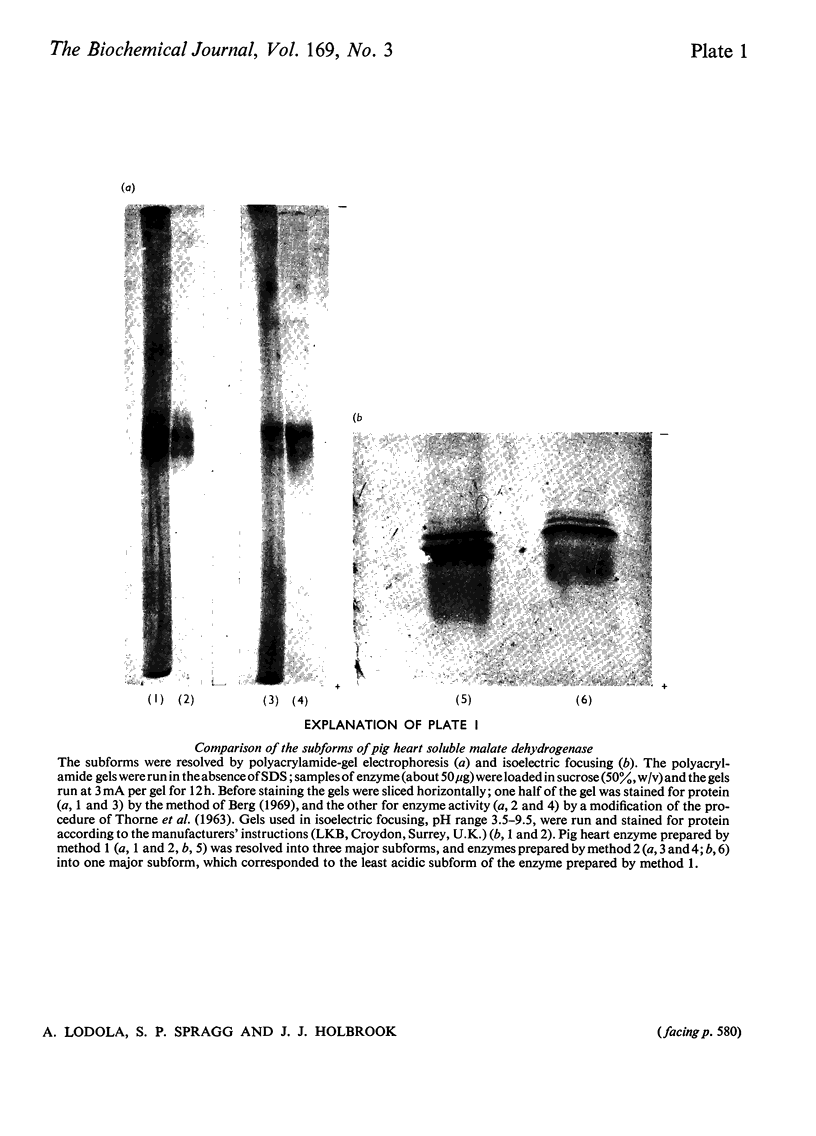
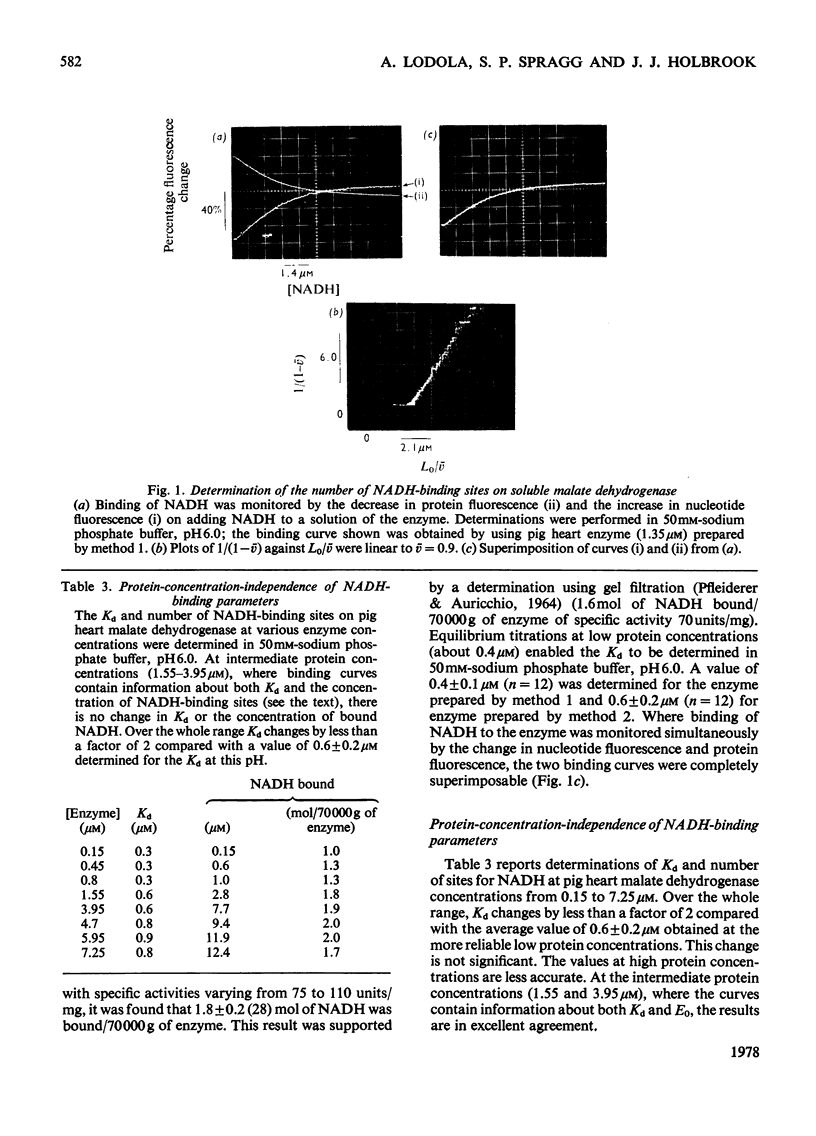
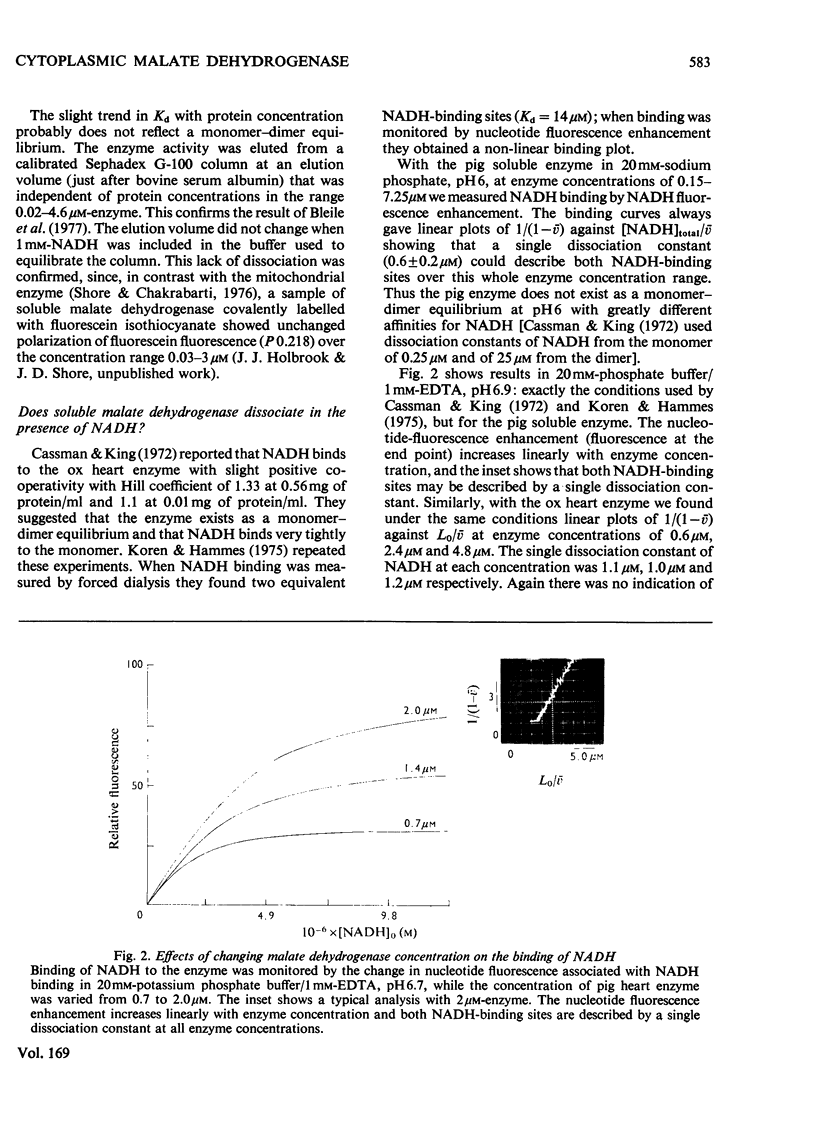
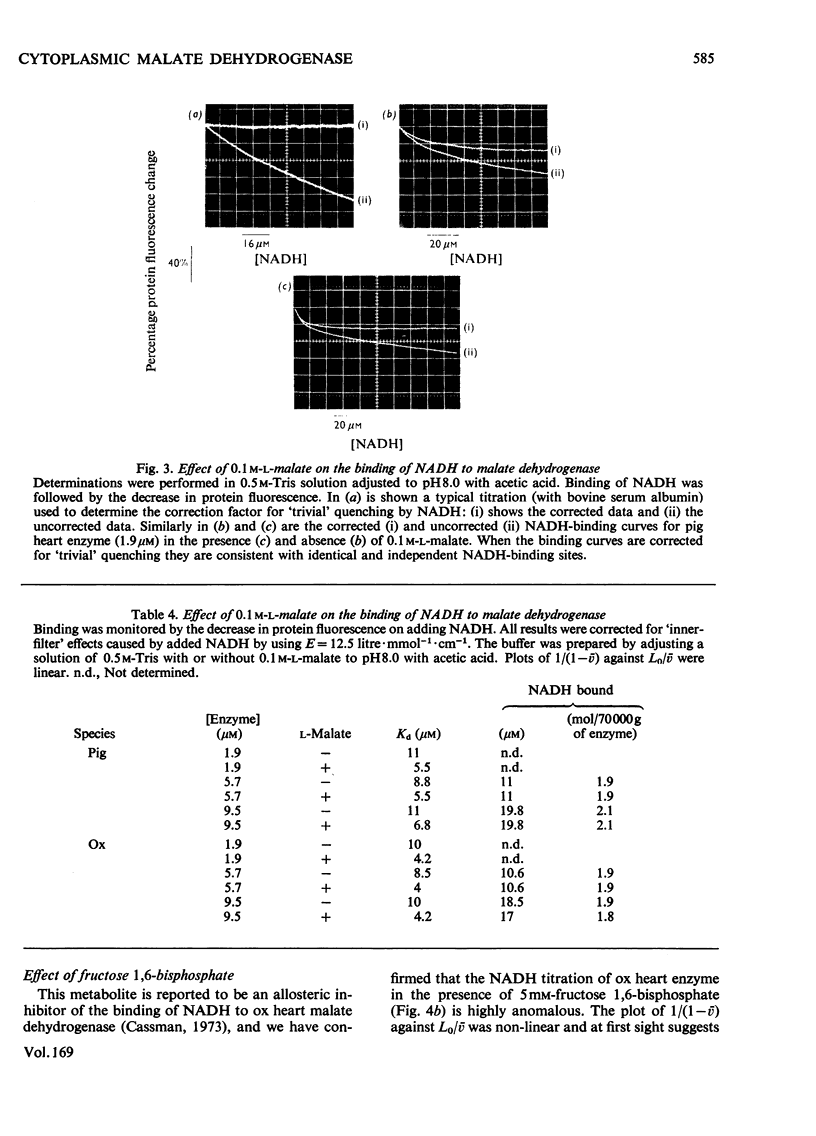
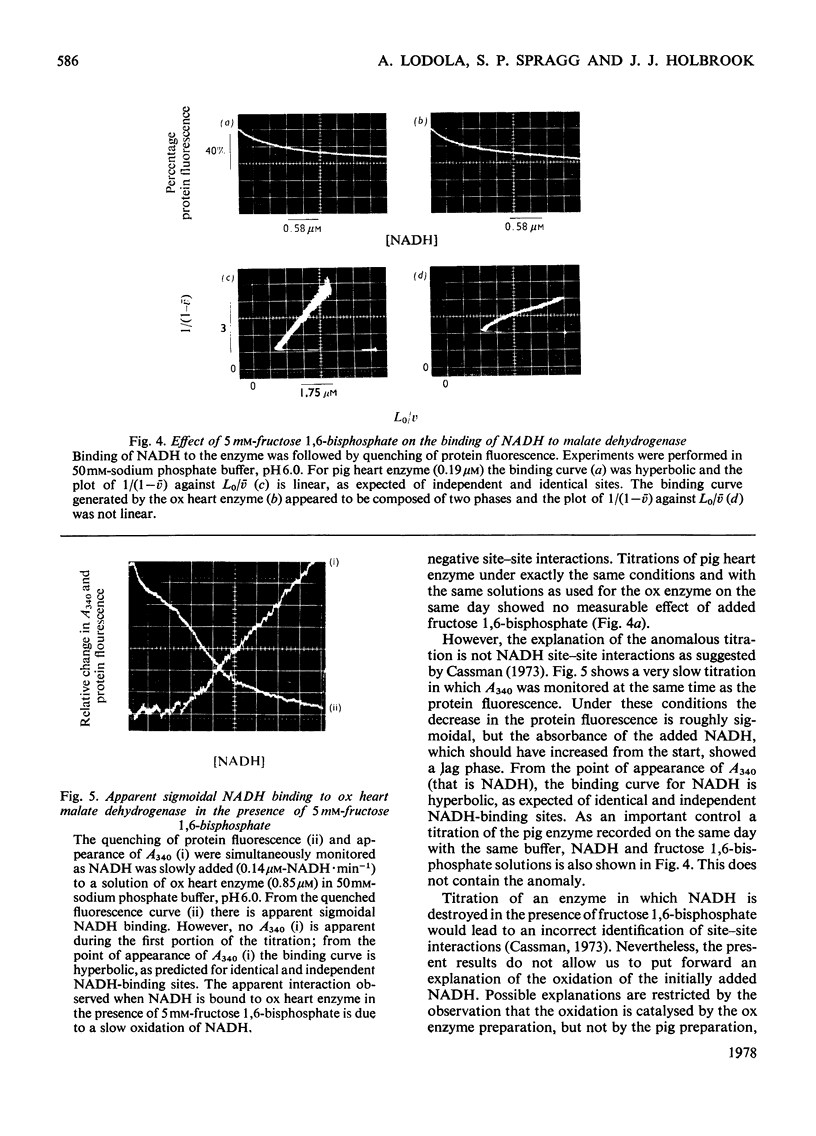
1. Two methods of preparing pig heart soluble malate dehydrogenase are described. A slow method yields an enzyme composed of three electrophoretically separable subforms. The more rapid method reproducibly gives a high yield of an enzyme that consists predominantly of the least acid subform. 2. The A1%1cm of the protein was redetermined as 15 at 280nm. By using this value the enzyme molecule was found to contain two independent and indistinguishable NADH-binding sites in titrations with NADH. 3. No evidence was found for the dissociation of the enzyme in the concentration range 0.02–7.2μm. 4. l-Malate (0.1m) tightened the binding of NADH to both pig and ox heart enzyme (2-fold), but, in contrast with the report by Mueggler, Dahlquist & Wolfe [(1975) Biochemistry 14, 3490–3497], did not cause co-operative interactions between the binding sites. 5. Fructose 1,6-bisphosphate had no effect on the binding of NADH to the pig heart enzyme, but with the ox heart enzyme the NADH is slowly oxidized. This slow oxidation explains the `sigmoidal' binding curves obtained when NADH was added to ox heart soluble malate dehydrogenase in the presence of fructose 1,6-bisphosphate [Cassman (1973) Biochem. Biophys. Res. Commun. 53, 666–672] without the postulate of site–site interactions. 6. It is concluded that neither l-malate nor fructose 1,6-bisphosphate could in vivo modulate the activity of soluble malate dehydrogenase and alter the rates of transport of NADH between the cytosol and the mitochondrion. 7. Details of the preparation of soluble malate dehydrogenase have been deposited as Supplementary Publication SUP 50080 (8 pages) at the British Library Lending Division, Boston Spa, Wetherby, West Yorkshire LS23 7BQ, U.K., from whom copies may be obtained under the terms given in Biochem. J. (1978) 169, 5.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
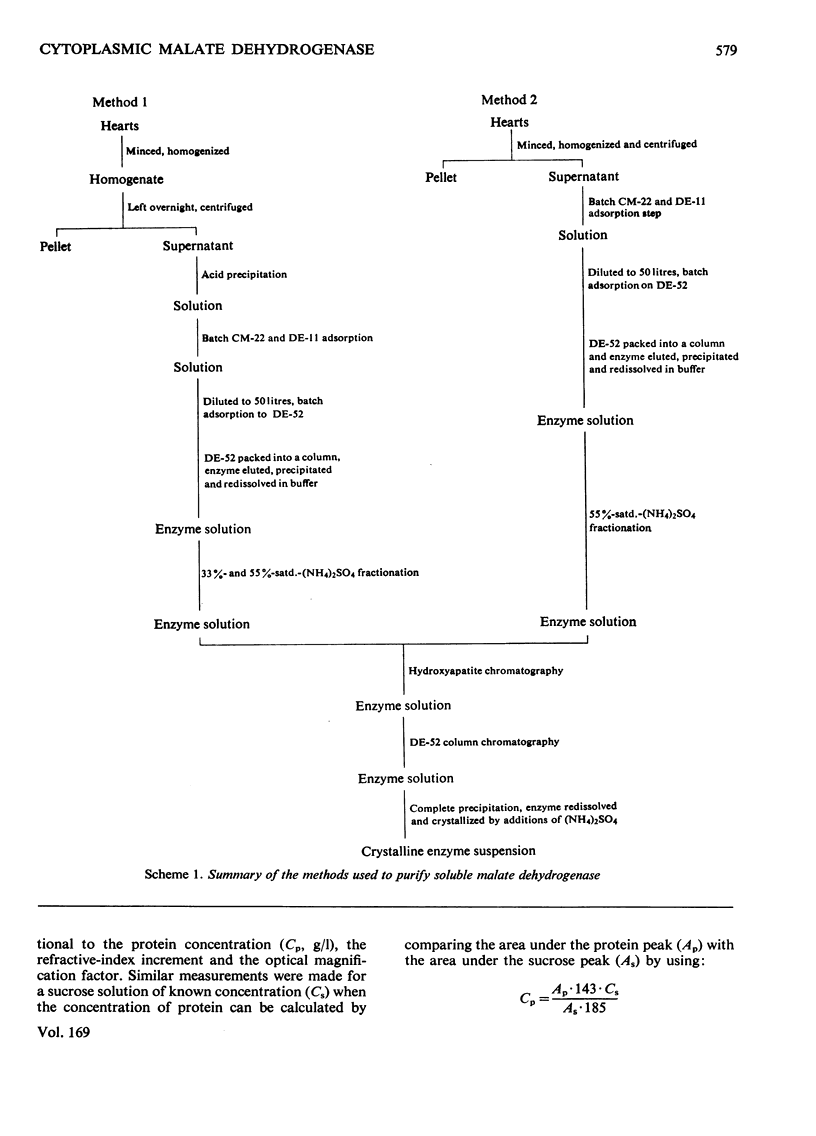
- Allen R. J. The estimation of phosphorus. Biochem J. 1940 Jun;34(6):858–865. doi: 10.1042/bj0340858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg H. C. Sulfanilic acid diazonium salt: a label for the outside of the human erythrocyte membrane. Biochim Biophys Acta. 1969 Jun 3;183(1):65–78. doi: 10.1016/0005-2736(69)90130-8. [DOI] [PubMed] [Google Scholar]
- Bleile D. M., Schulz R. A., Harrison J. H., Gregory E. M. Investigation of the subunit interactions in malate dehydrogenase. J Biol Chem. 1977 Jan 25;252(2):755–758. [PubMed] [Google Scholar]
- Cassman M. Allosteric and isosteric modifiers of NADH binding to cytoplasmic malic dehydrogenase. Biochem Biophys Res Commun. 1973 Jul 17;53(2):666–672. doi: 10.1016/0006-291x(73)90713-4. [DOI] [PubMed] [Google Scholar]
- Cassman M., King R. C. Subunit interactions and ligand binding in supernatant malic dehydrogenase. Cooperative binding of reduced nicotinamide adenine dinucleotide associated with a monomer-dimer equilibrium of the protein. Biochemistry. 1972 Dec 19;11(26):4937–4944. doi: 10.1021/bi00776a010. [DOI] [PubMed] [Google Scholar]
- Cassman M., Vetterlein D. Allosteric and nonallosteric interactions with reduced nicotinamide adenine dinucleotide in two forms of cytoplasmic malic dehydrogenase. Biochemistry. 1974 Feb 12;13(4):684–689. doi: 10.1021/bi00701a008. [DOI] [PubMed] [Google Scholar]
- Frieden C., Fernandez-Sousa J. Kinetic studies on pig heart cytoplasmic malate dehydrogenase. J Biol Chem. 1975 Mar 25;250(6):2106–2113. [PubMed] [Google Scholar]
- Gerding R. K., Wolfe R. G. Malic dehydrogenase. 8. Large scale purification and properties of supernatant pig heart enzyme. J Biol Chem. 1969 Mar 10;244(5):1164–1171. [PubMed] [Google Scholar]
- Glatthaar B. E., Banaszak L. J., Bradshaw R. A. The identification of an asymmetric complex of nicotinamide adenine dinucleotid and pig heart cytoplasmic malate dehydrogenase. Biochem Biophys Res Commun. 1972 Jan 31;46(2):757–765. doi: 10.1016/s0006-291x(72)80205-5. [DOI] [PubMed] [Google Scholar]
- Hill E., Tsernoglou D., Webb L., Banaszak L. J. Polypeptide conformation of cytoplasmic malate dehydrogenase from an electron density map at 3.0 angstrom resolution. J Mol Biol. 1972 Dec 30;72(3):577–589. doi: 10.1016/0022-2836(72)90176-3. [DOI] [PubMed] [Google Scholar]
- Holbrook J. J. Protein fluorescence of lactate dehydrogenase. Biochem J. 1972 Jul;128(4):921–931. doi: 10.1042/bj1280921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook J. J., Wolfe R. G. Malate dehydrogenase. X. Fluorescence microtitration studies of D-malate, hydroxymalonate, nicotinamide dinucleotide, and dihydronicotinamide-adenine dinucleotide binding by mitochondrial and supernatant porcine heart enzymes. Biochemistry. 1972 Jun 20;11(13):2499–2502. doi: 10.1021/bi00763a018. [DOI] [PubMed] [Google Scholar]
- Koren R., Hammes G. G. Interaction of reduced nicotinamide adenine dinucleotide with beef heart s-malate dehydrogenase. Biochemistry. 1975 Mar 11;14(5):1021–1025. doi: 10.1021/bi00676a021. [DOI] [PubMed] [Google Scholar]
- Mueggler P. A., Dahlquist F. W., Wolfe R. G. Malate dehydrogenase, anticooperative NADH, and L-malate binding in ternary complexes with Supernatant pig heart enzyme. Biochemistry. 1975 Jul 29;14(15):3490–3497. doi: 10.1021/bi00686a031. [DOI] [PubMed] [Google Scholar]
- Pfleiderer G., Auricchio F. The DPNH-binding capacity of various dehydrogenases. Biochem Biophys Res Commun. 1964 May 22;16(1):53–59. doi: 10.1016/0006-291x(64)90210-4. [DOI] [PubMed] [Google Scholar]
- Shore J. D., Chakrabarti S. K. Subunit dissociation of mitochondrial malate dehydrogenase. Biochemistry. 1976 Feb 24;15(4):875–879. doi: 10.1021/bi00649a023. [DOI] [PubMed] [Google Scholar]
- Stinson R. A., Holbrook J. J. Equilibrium binding of nicotinamide nucleotides to lactate dehydrogenases. Biochem J. 1973 Apr;131(4):719–728. doi: 10.1042/bj1310719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THORNE C. J., GROSSMAN L. I., KAPLAN N. O. Starch-gel electrophoresis of malate dehydrogenase. Biochim Biophys Acta. 1963 Jun 11;73:193–203. doi: 10.1016/0006-3002(63)90303-2. [DOI] [PubMed] [Google Scholar]
- Tanner M. J., Boxer D. H. Separation and some properties of the major proteins of the human erythrocyte membrane. Biochem J. 1972 Sep;129(2):333–347. doi: 10.1042/bj1290333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson J. R., Safer B., LaNoue K. F., Smith C. M., Walajtys E. Mitochondrial-cytosolic interactions in cardiac tissue: role of the malate-aspartate cycle in the removal of glycolytic NADH from the cytosol. Symp Soc Exp Biol. 1973;27:241–281. [PubMed] [Google Scholar]