Abstract
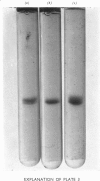
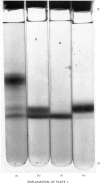
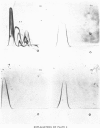
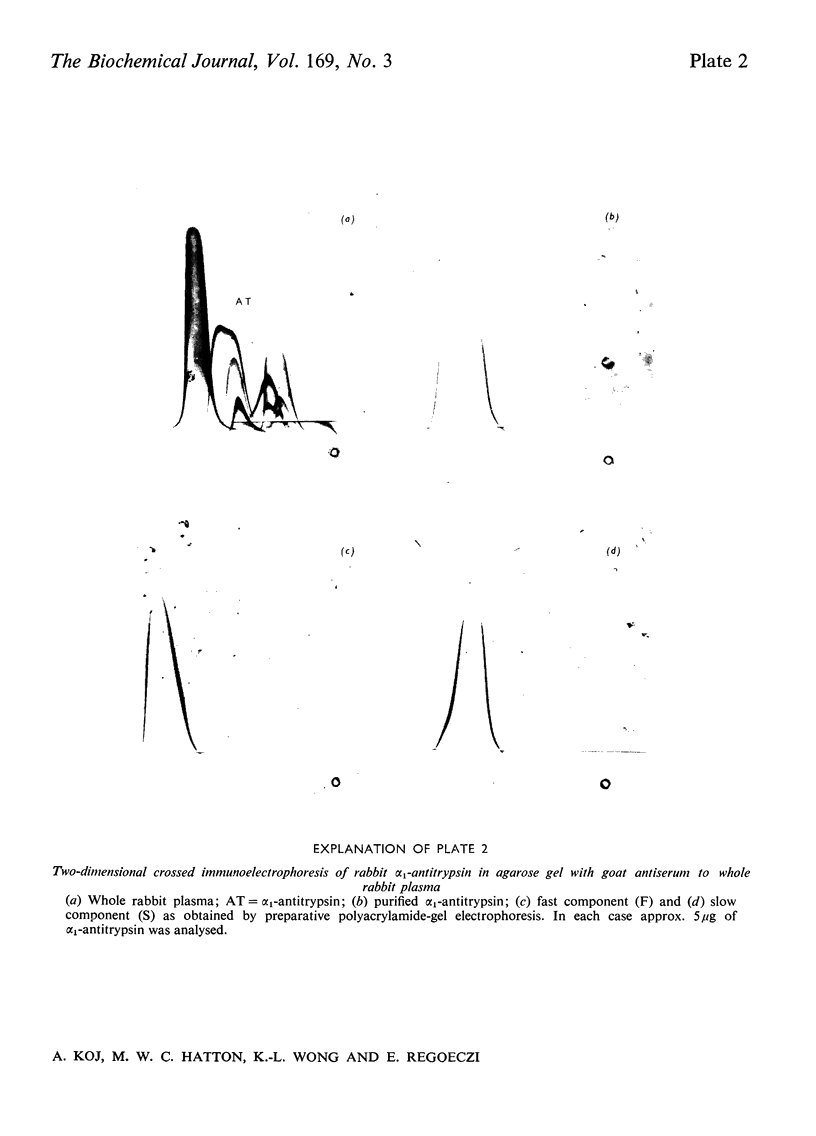
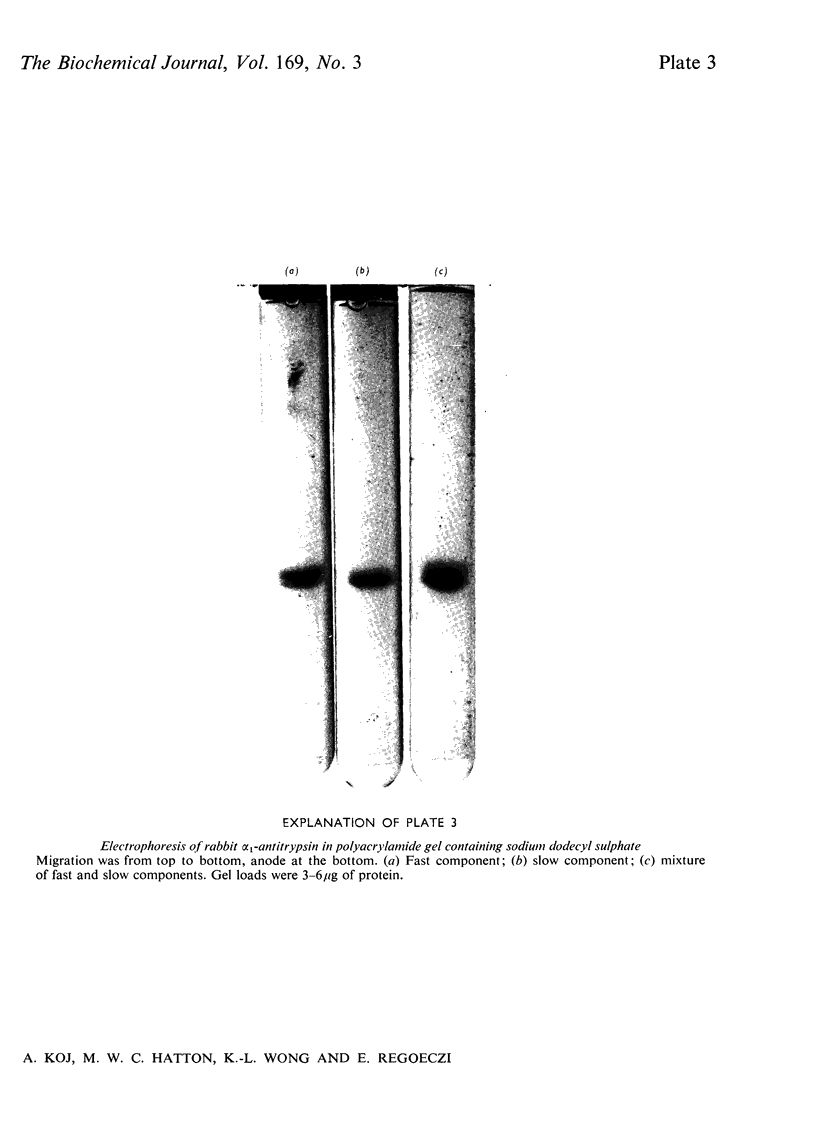
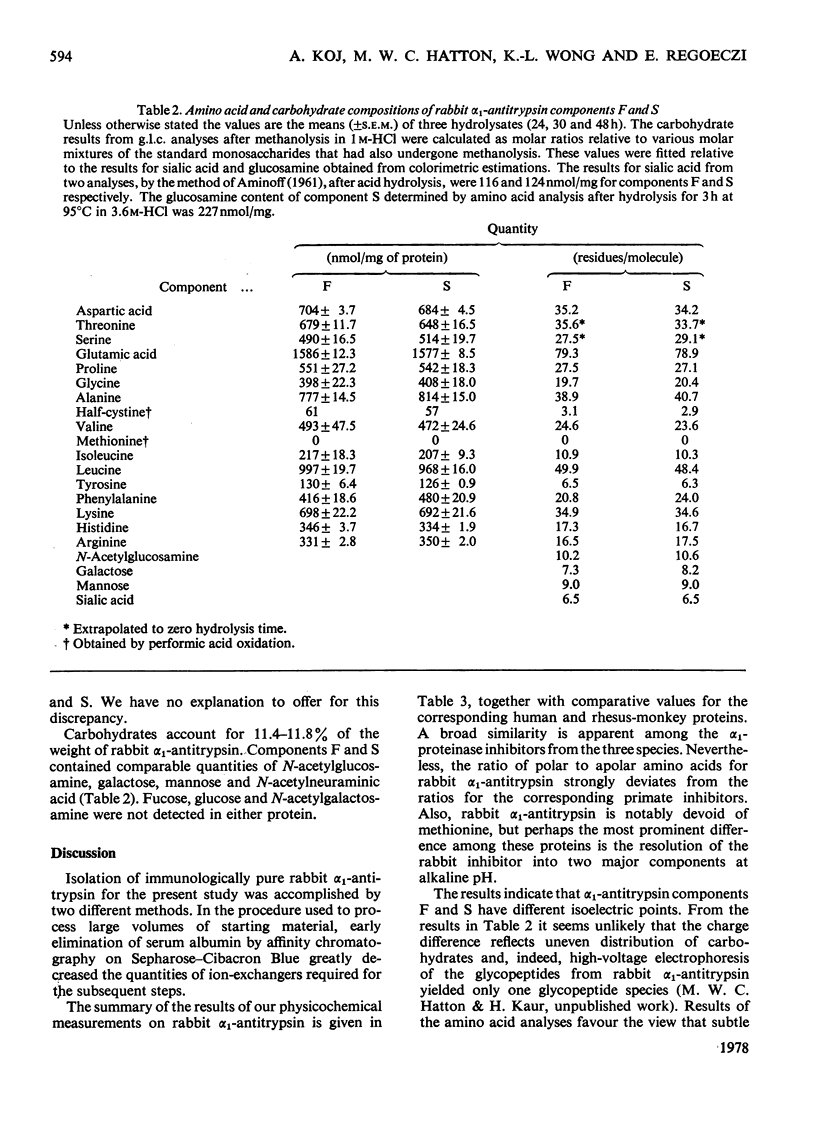
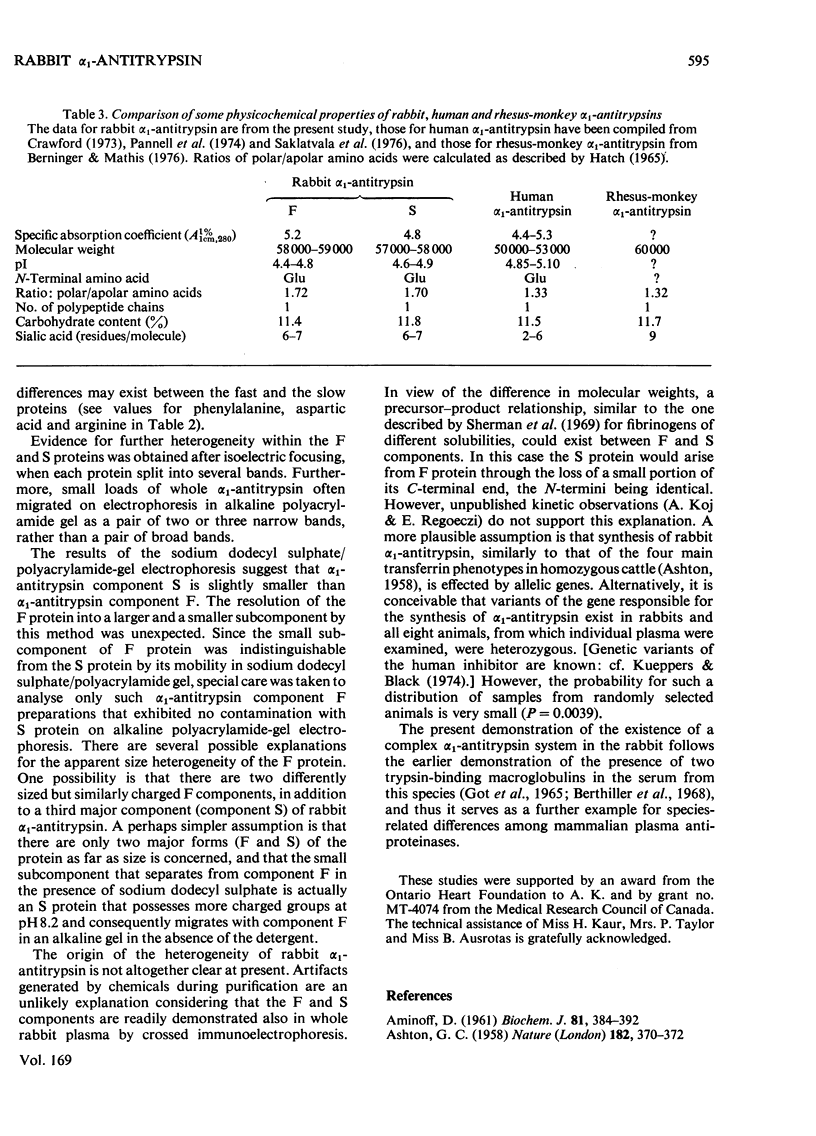
Alpha1-Antitrypsin was isolated from rabbit plasma by salting out with (NH4)2SO4 followed by ion-exchange chromatography either on DEAE-Sephadex or DEAE-cellulose (each at pH8.8 and 6.5), and affinity chromatography on Sepharose-Cibacron Blue and Sepharose-concanavalin A. The protein thus obtained was homogeneous during crossed immunoelectrophoresis by using an antiserum to whole rabbit plasma, but it migrated as two broad bands when electrophoresed in alkaline polyacrylamide gels. Under optimal loading conditions, two or three subcomponents could be distinguished in each band. The two major forms of rabbit alpha1-antitrypsin, designated components F and S, were separated by preparative polyacrylamide-gel electrophoresis, and some of their physico-chemical properties were established. Both forms reacted with trypsin at a molar ratio of 1:1. Their elution volumes from a Sephadex G-200 column were identical, corresponding to a mol.wt. of 58000; however, some heterogeneity was observed after sodium dodecyl sulphate/polyacrylamide-gel electrophoresis. Isoelectric focusing in polyacrylamide gel in a pH 4-6 gradient revealed a multiple-band pattern for each form in the range of pH4.4-4.9. The two forms of rabbit alpha1-antitrypsin possessed the same N-terminal amino acid (glutamic acid) and had very similar amino acid and carbohydrate compositions.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMINOFF D. Methods for the quantitative estimation of N-acetylneuraminic acid and their application to hydrolysates of sialomucoids. Biochem J. 1961 Nov;81:384–392. doi: 10.1042/bj0810384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASHTON G. C. Genetics of beta-globulin polymorphism in British cattle. Nature. 1958 Aug 9;182(4632):370–372. doi: 10.1038/182370a0. [DOI] [PubMed] [Google Scholar]
- BEAVEN G. H., HOLIDAY E. R. Ultraviolet absorption spectra of proteins and amino acids. Adv Protein Chem. 1952;7:319–386. doi: 10.1016/s0065-3233(08)60022-4. [DOI] [PubMed] [Google Scholar]
- Berninger R. W., Mathis R. K. Isolation and characterization of alpha-1-antitrypsin from rhesus-monkey serum. Biochem J. 1976 Oct 1;159(1):95–104. doi: 10.1042/bj1590095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthillier G., Got R., Bertagnolio G. Biochimie de l'alpha 1-macroglobuline de lapin. IV. Effet sur l'activité estérasique de la trypsine et de la chymotrypsine. Biochim Biophys Acta. 1968 Nov 12;170(1):140–151. [PubMed] [Google Scholar]
- CLARKE J. T. SIMPLIFIED "DISC" (POLYACRYLAMIDE GEL) ELECTROPHORESIS. Ann N Y Acad Sci. 1964 Dec 28;121:428–436. doi: 10.1111/j.1749-6632.1964.tb14214.x. [DOI] [PubMed] [Google Scholar]
- Chase T., Jr, Shaw E. p-Nitrophenyl-p'-guanidinobenzoate HCl: a new active site titrant for trypsin. Biochem Biophys Res Commun. 1967 Nov 30;29(4):508–514. doi: 10.1016/0006-291x(67)90513-x. [DOI] [PubMed] [Google Scholar]
- Crawford I. P. Purification and properties of normal human alpha 1-antitrypsin. Arch Biochem Biophys. 1973 May;156(1):215–222. doi: 10.1016/0003-9861(73)90359-7. [DOI] [PubMed] [Google Scholar]
- GRAY W. R., HARTLEY B. S. THE STRUCTURE OF A CHYMOTRYPTIC PEPTIDE FROM PSEUDOMONAS CYTOCHROME C-551. Biochem J. 1963 Nov;89:379–380. doi: 10.1042/bj0890379. [DOI] [PubMed] [Google Scholar]
- Glaser C. B., Karic L., Cohen A. B. Low pH stability of alpha-1-antititrypsin. Biochim Biophys Acta. 1977 Mar 28;491(1):325–330. doi: 10.1016/0005-2795(77)90068-x. [DOI] [PubMed] [Google Scholar]
- Glaser C. B., Karic L., Fallat R. Isolation and characterization of alpha-1-antitrypsin from the Cohn fraction IV-I of human plasma. Prep Biochem. 1975;5(4):333–348. doi: 10.1080/00327487508061581. [DOI] [PubMed] [Google Scholar]
- Gordon A. H. The alpha macroglobulins of rat serum. Biochem J. 1976 Dec 1;159(3):643–650. doi: 10.1042/bj1590643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Got R., Mouray H., Moretti J. Etude biochimique de l'alpha 1-macroglobuline du Sérum de lapin. I. Préparation et propriétés physico-chimiques. Biochim Biophys Acta. 1965 Sep 13;107(2):278–285. [PubMed] [Google Scholar]
- Hatch F. T. Correlation of amino-acid composition with certain characteristics of proteins. Nature. 1965 May 22;206(4986):777–779. doi: 10.1038/206777a0. [DOI] [PubMed] [Google Scholar]
- Hercz A., Barton M. The purification and properties of human alpha1-antitrypsin (alpha1-antiprotease), variant Z. Eur J Biochem. 1977 Apr 15;74(3):603–610. doi: 10.1111/j.1432-1033.1977.tb11429.x. [DOI] [PubMed] [Google Scholar]
- Kress L. F., Laskowski M., Sr Large scale purification of alpha-1 trypsin inhibitor from human plasma. Prep Biochem. 1973;3(6):541–552. doi: 10.1080/00327487308061536. [DOI] [PubMed] [Google Scholar]
- Kueppers F., Black L. F. Alpha1-antitrypsin and its deficiency. Am Rev Respir Dis. 1974 Aug;110(2):176–194. doi: 10.1164/arrd.1974.110.2.176. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McFARLANE A. S. Efficient trace-labelling of proteins with iodine. Nature. 1958 Jul 5;182(4627):53–53. doi: 10.1038/182053a0. [DOI] [PubMed] [Google Scholar]
- Miller R. R., Kuhlenschmidt M. S., Coffee C. J., Kuo I., Glew R. H. Comparison of the chemical, physical, and survival properties of normal and Z-variant alpha-1-antitrypsins. J Biol Chem. 1976 Aug 10;251(15):4751–4757. [PubMed] [Google Scholar]
- OPIENSKA-BLAUTH J., CHAREZINSKI M., BERBEC H. A new, rapid method of determining tryptophan. Anal Biochem. 1963 Jul;6:69–76. doi: 10.1016/0003-2697(63)90009-5. [DOI] [PubMed] [Google Scholar]
- Pajdak W. A simple apparatus for preparative electrophoresis in polyacrylamide gel. Clin Chim Acta. 1973 Sep 28;48(1):113–115. doi: 10.1016/0009-8981(73)90225-8. [DOI] [PubMed] [Google Scholar]
- Pannell R., Johnson D., Travis J. Isolation and properties of human plasma alpha-1-proteinase inhibitor. Biochemistry. 1974 Dec 17;13(26):5439–5445. doi: 10.1021/bi00723a031. [DOI] [PubMed] [Google Scholar]
- Regoeczi E., Wong K. L., Ali M., Hatton M. W. The molecular components of human transferrin type C. Int J Pept Protein Res. 1977;10(1):17–26. doi: 10.1111/j.1399-3011.1977.tb02772.x. [DOI] [PubMed] [Google Scholar]
- Robinson N. C., Tye R. W., Neurath H., Walsh K. A. Isolation of trypsins by affinity chromatography. Biochemistry. 1971 Jul 6;10(14):2743–2747. doi: 10.1021/bi00790a014. [DOI] [PubMed] [Google Scholar]
- Saklatvala J., Wood G. C., White D. D. Isolation and characterization of human plasma alpha 1-proteinase inhibitor and a conformational study of its interaction with proteinases. Biochem J. 1976 Aug 1;157(2):339–351. doi: 10.1042/bj1570339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman L. A., Fletcher A. P., Sherry S. In vivo transformation between fibrinogens of varying ethanol solubilities: a pathway of fibrinogen catabolism. J Lab Clin Med. 1969 Apr;73(4):574–583. [PubMed] [Google Scholar]
- Travis J., Bowen J., Tewksbury D., Johnson D., Pannell R. Isolation of albumin from whole human plasma and fractionation of albumin-depleted plasma. Biochem J. 1976 Aug 1;157(2):301–306. doi: 10.1042/bj1570301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]
- Woods K. R., Wang K. T. Separation of dansyl-amino acids by polyamide layer chromatography. Biochim Biophys Acta. 1967 Feb 21;133(2):369–370. doi: 10.1016/0005-2795(67)90078-5. [DOI] [PubMed] [Google Scholar]