Abstract
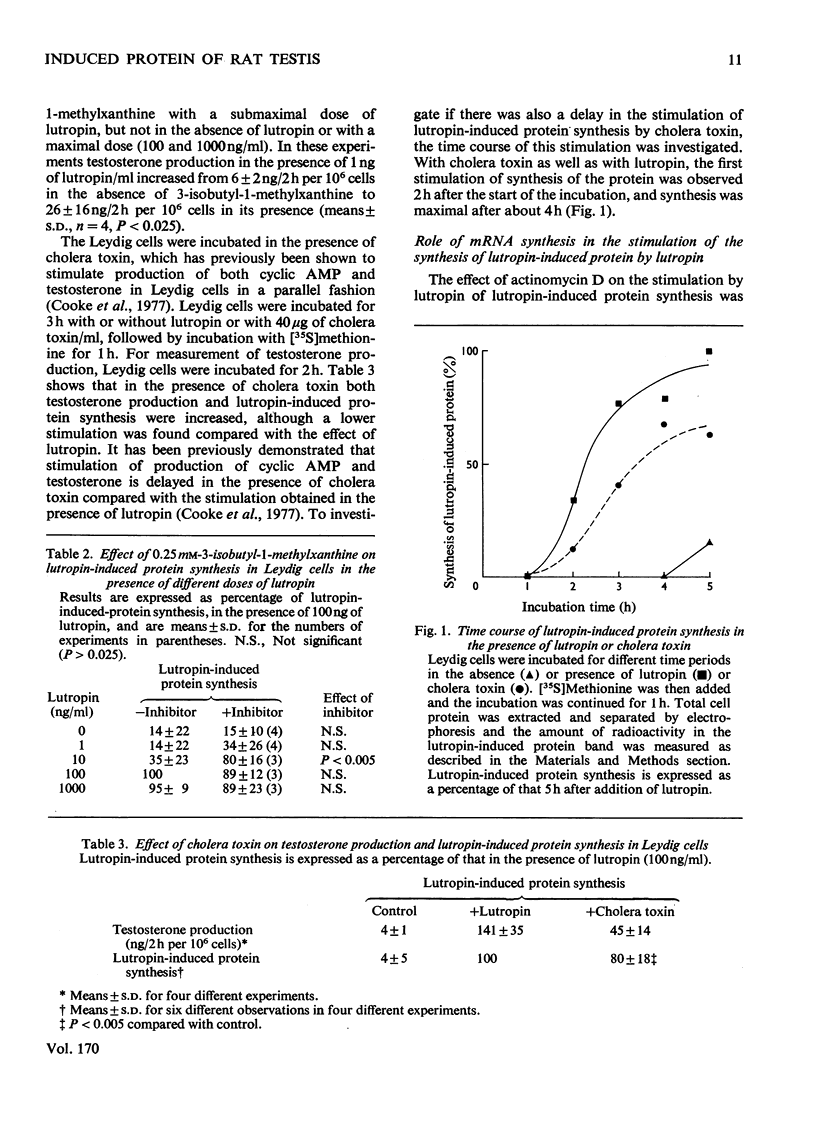
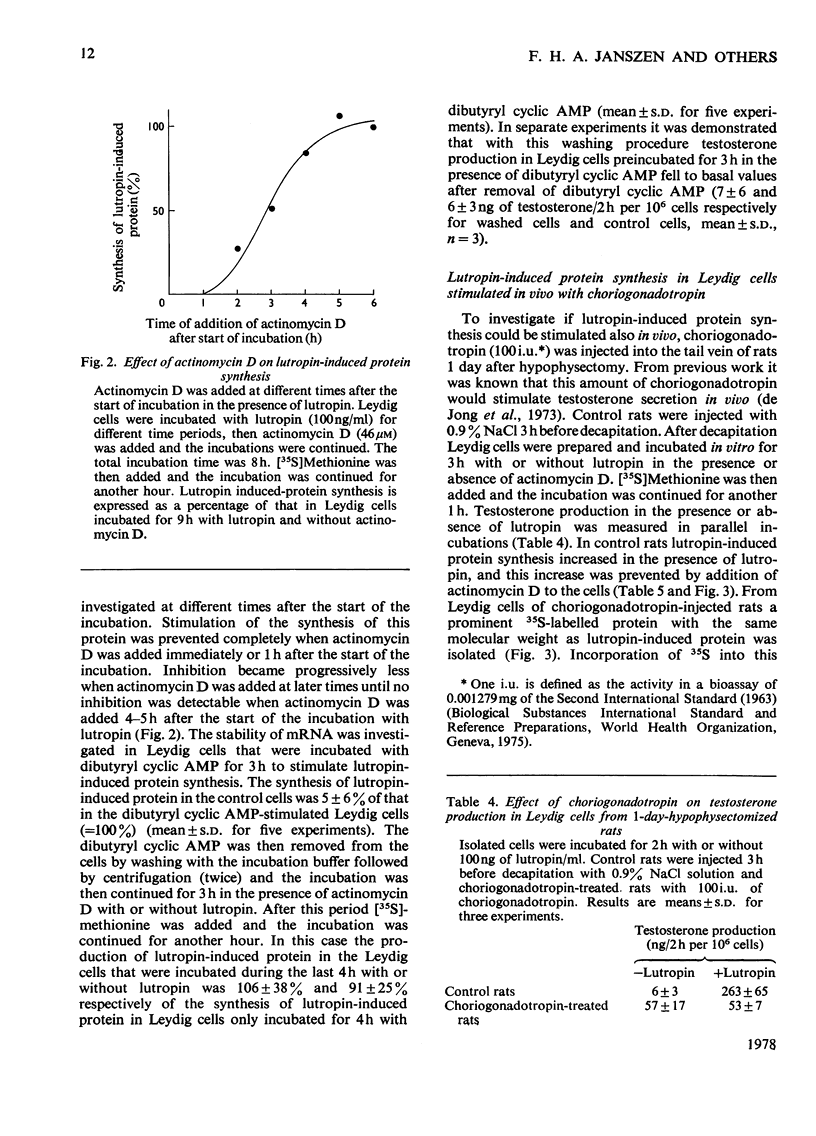
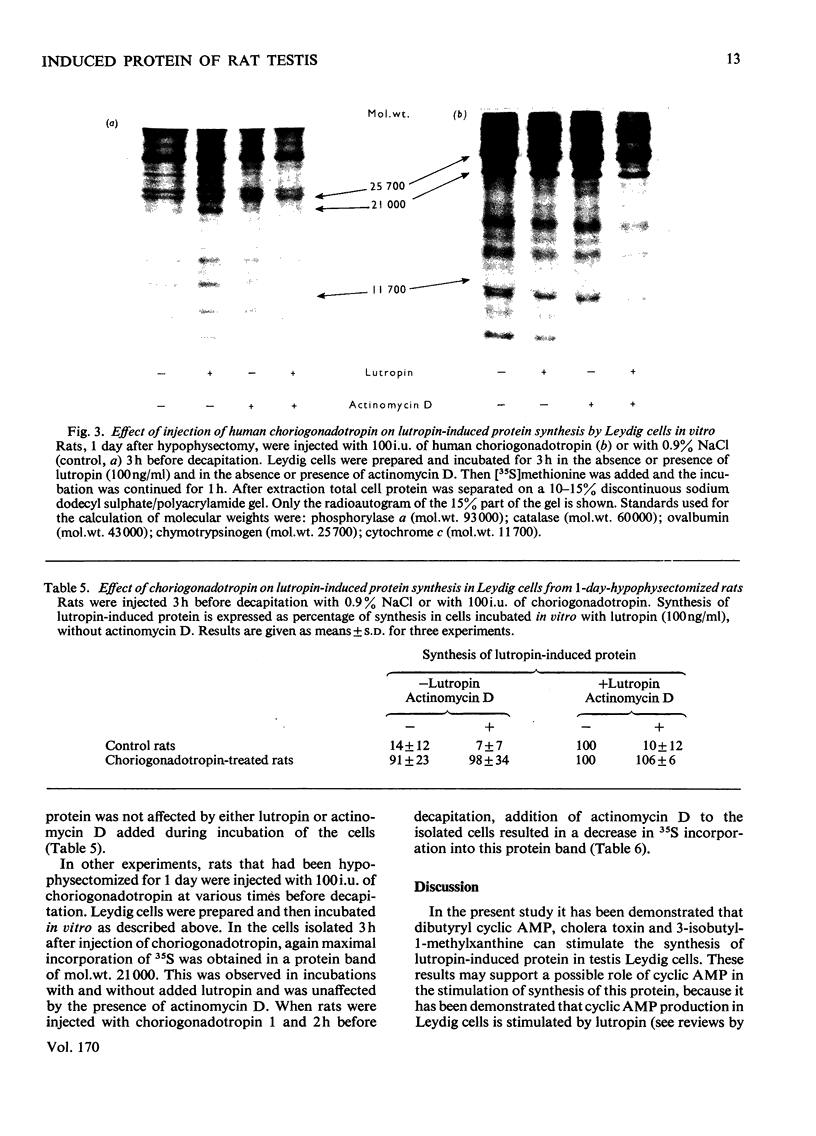
The mechanism of action of lutropin on the stimulation of the synthesis of a specific lutropin-induced protein in rat testis Leydig cells was investigated. Lutropin-induced protein has a mol.wt. of approx. 21000 and is detected by labelling the Leydig-cell proteins with [35S]methionine, followed by separation by polyacrylamide-gel electrophoresis and radioautography of the dried gel. The incorporation of 35S into lutropin-induced protein was used as an estimate for the synthesis of the protein. Incubation of Leydig cells with dibutyryl cyclic AMP or cholera toxin also resulted in the stimulation of synthesis of the protein. Synthesis of lutropin-induced protein, when maximally stimulated with 100ng of lutropin/ml, could not be stimulated further by addition of dibutyryl cyclic AMP. Addition of 3-isobutyl-1-methylxanthine, a phosphodiesterase inhibitor, further increased synthesis of the protein in the presence of a submaximal dose of lutropin (10ng/ml) but not in the absence of lutropin or with maximal amounts of lutropin (100 and 1000ng/ml). Actinomycin D prevented the effect of lutropin on the stimulation of lutropin-induced protein synthesis when added immediately or 1h after the start of the incubation, but not when added after 5–6h. This is interpreted as reflecting that, after induction of mRNA coding for lutropin-induced protein, lutropin had no influence on the synthesis of the protein in the presence of actinomycin D. Synthesis of the protein was also stimulated in vivo by injection of choriogonadotropin into rats 1 day after hypophysectomy, and the time course of this stimulation of lutropin-induced protein synthesis in vivo was similar to that obtained by incubating Leydig cells in vitro with lutropin. From these results it is concluded that stimulation of lutropin-induced protein synthesis by lutropin is most probably mediated by cyclic AMP and involves synthesis of mRNA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Catt K. J., Dufau M. L. Basic concepts of the mechanism of action of peptide hormones. Biol Reprod. 1976 Feb;14(1):1–15. doi: 10.1095/biolreprod14.1.1. [DOI] [PubMed] [Google Scholar]
- Catt K. J., Tsuruhara T., Mendelson C., Ketelslegers J. M., Dufau M. L. Gonadotroping binding and activation of the interstitial cells of the testis. Curr Top Mol Endocrinol. 1974;1:1–30. doi: 10.1007/978-1-4684-2595-6_1. [DOI] [PubMed] [Google Scholar]
- Catt K. J., Watanabe K., Dufau M. L. Cyclic AMP released by rat testis during gonadotrophin stimulation in vitro. Nature. 1972 Sep 29;239(5370):280–281. doi: 10.1038/239280a0. [DOI] [PubMed] [Google Scholar]
- Cooke B. A., Janszen F. H., Clotscher W. F., van der Molen H. J. Effect of protein-synthesis inhibitors on testosterone production in rat testis interstitial tissue and Leydig-cell preparations. Biochem J. 1975 Sep;150(3):413–418. doi: 10.1042/bj1500413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke B. A., Lindh L. M., Janszen F. H. Role of cyclic AM in steroidogenesis in Leydig cells: discrepancies' between effects of luteinizing hormone and cholera toxin. FEBS Lett. 1977 Jan 15;73(1):67–71. [PubMed] [Google Scholar]
- HALL P. F., EIK-NES K. B. The action of gonadotropic hormones upon rabbit testis in vitro. Biochim Biophys Acta. 1962 Oct 8;63:411–422. doi: 10.1016/0006-3002(62)90105-1. [DOI] [PubMed] [Google Scholar]
- Hsueh A. J., Dufau M. L., Katz S. I., Catt K. J. Immunofluorescence labeling of gonadotropin receptors in enzyme-dispersed interstitial cells. Nature. 1976 Jun 24;261(5562):710–712. doi: 10.1038/261710a0. [DOI] [PubMed] [Google Scholar]
- Janszen F. H., Cooke B. A., van Driel M. J., van der Molen H. J. LH induction of a specific protein (LH-IP) in rat testis Leydig cells. FEBS Lett. 1976 Dec 1;71(2):269–272. doi: 10.1016/0014-5793(76)80948-9. [DOI] [PubMed] [Google Scholar]
- Janszen F. H., Cooke B. A., van Driel M. J., van der Molen H. J. Purification and characterization of Leydig cells from rat testes. J Endocrinol. 1976 Sep;70(3):345–359. doi: 10.1677/joe.0.0700345. [DOI] [PubMed] [Google Scholar]
- Janszen F. H., Cooke B. A., van der Molen H. J. Specific protein synthesis in isolated rat testis leydig cells. Influence of luteinizing hormone and cycloheximide. Biochem J. 1977 Feb 15;162(2):341–346. doi: 10.1042/bj1620341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh J. M. The role of cyclic AMP in gonadal steroidogenesis. Biol Reprod. 1976 Feb;14(1):30–53. doi: 10.1095/biolreprod14.1.30. [DOI] [PubMed] [Google Scholar]
- Mendelson C., Dufau M., Catt K. Dependence of gonadotropin-induced steroidogenesis upon RNA and protein synthesis in the interstitial cells of the rat testis. Biochim Biophys Acta. 1975 Dec 5;411(2):222–230. doi: 10.1016/0304-4165(75)90302-5. [DOI] [PubMed] [Google Scholar]
- Moyle W. R., Ramachandran J. Effect of LH on steroidogenesis and cyclic AMP accumulation in rat Leydig cell preparations and mouse tumor Leydig cells. Endocrinology. 1973 Jul;93(1):127–134. doi: 10.1210/endo-93-1-127. [DOI] [PubMed] [Google Scholar]
- Rommerts F. F., Cooke B. A., van der Molen H. J. The role of cyclic AMP in the regulation of steroid biosynthesis in testis tissue. J Steroid Biochem. 1974 May;5(3):279–285. doi: 10.1016/0022-4731(74)90143-5. [DOI] [PubMed] [Google Scholar]
- Verjans H. L., Cooke B. A., de Jong F. H., de Jong C. M., van der Molen H. J. Evaluation of a radioimmunoassay for testosterone estimation. J Steroid Biochem. 1973 Nov;4(6):665–676. doi: 10.1016/0022-4731(73)90042-3. [DOI] [PubMed] [Google Scholar]
- de Jong F. H., Hey A. H., van der Molen H. J. Effect of gonadotrophins on the secretion of oestradiol- and testosterone by the rat testis. J Endocrinol. 1973 May;57(2):277–284. doi: 10.1677/joe.0.0570277. [DOI] [PubMed] [Google Scholar]



