Abstract
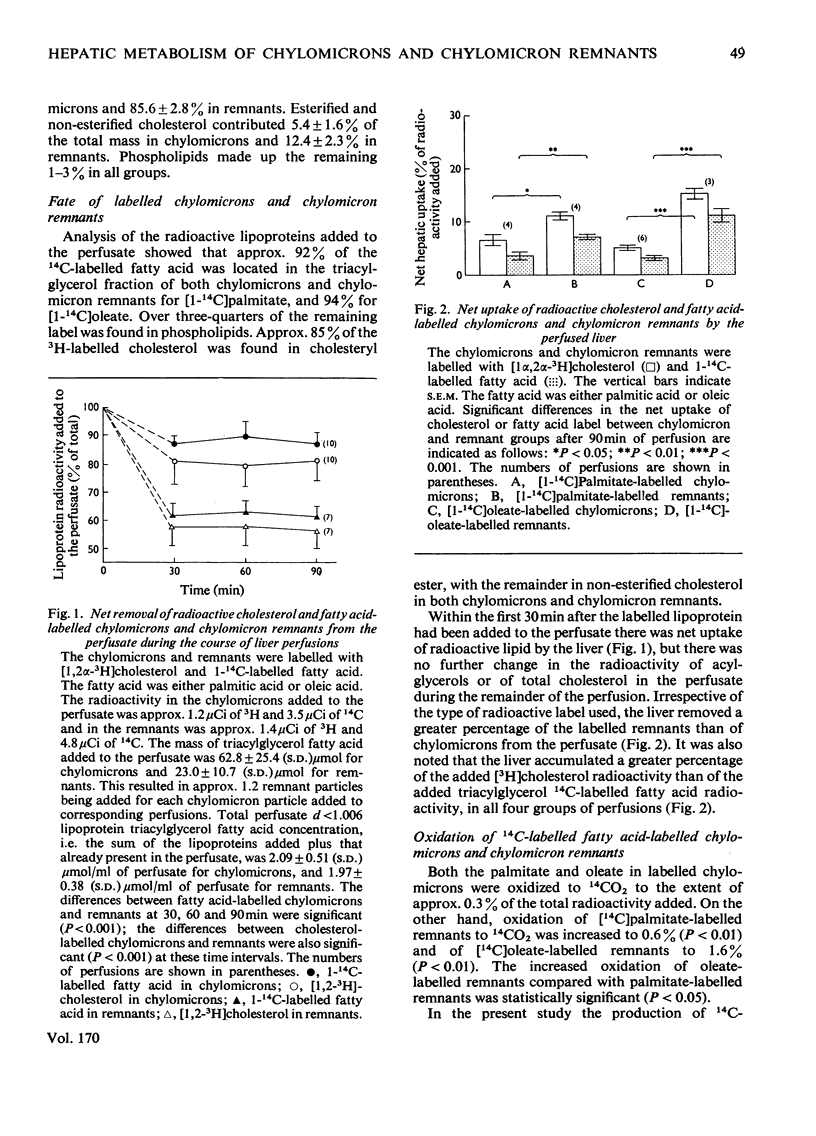
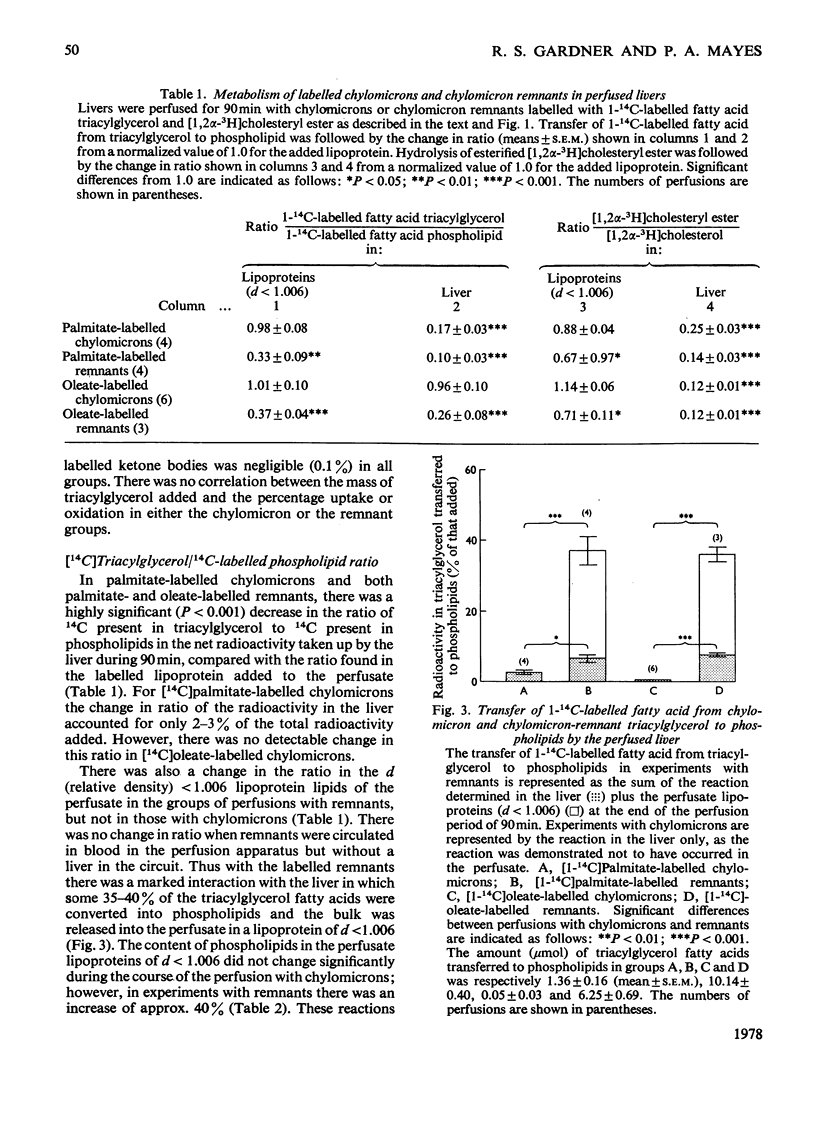
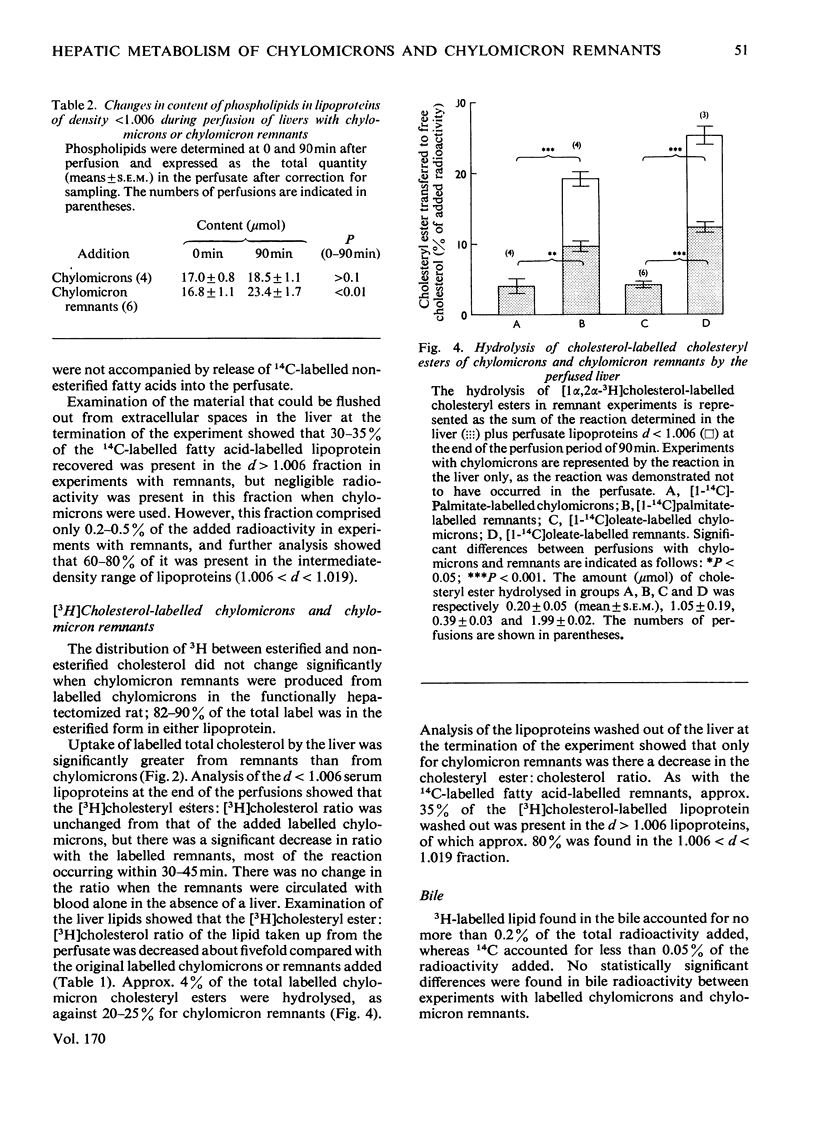
1. The hepatic metabolism of chylomicrons and chylomicron remnants was compared after adding approximately equal numbers of each lipoprotein particle to the perfusate of isolated livers. 2. At least 40% of the added remnants were metabolized by the liver compared with less than 3% for chylomicrons. 3. There was significantly more net removal of labelled remnants than of chylomicrons by the liver. 4. A greater proportion of labelled cholesterol than of labelled triacylglycerol fatty acids was transferred to the liver from each lipoprotein. 5. Cholesteryl esters of remnants were hydrolysed to triacylglycerol fatty lipoprotein. 5. Cholesteryl esters of remnants were hydrolysed to triacylglycerol fatty acids of remnants were oxidized to CO2 more extensively than those of chylomicrons. 6. There was greater oxidation of remnant glycerolipic [(1(-14)C]oleate than of glycerolipid [1(-14)C]palmitate. 7. A large fraction of the fatty acids of remnants, but not of chylomicrons, was transferred to phospholipids, which were released by the liver in a lipoprotein of relative density less than 1.006. 8. Label from remnants, but not from chylomicrons, was found in lipoproteins of relative density greater than 1.006, which were not released during perfusion but could be flushed out from the liver at the end of perfusion.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assmann G., Krauss R. M., Fredrickson D. S., Levy R. I. Characterization, subcellular localization, and partial purification of a heparin-released triglyceride lipase from rat liver. J Biol Chem. 1973 Mar 25;248(6):1992–1999. [PubMed] [Google Scholar]
- BRAGDON J. H., GORDON R. S., Jr Tissue distribution of C14 after the intravenous injection of labeled chylomicrons and unesterified fatty acids in the rat. J Clin Invest. 1958 Apr;37(4):574–578. doi: 10.1172/JCI103640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfrage P. Metabolism of chyle triglycerides in the liver. I. Studies on the mechanisms for liver uptake of intravenously injected, glycerol- and fatty acid-labeled chyle in the carbohydrate-fed rat. Biochim Biophys Acta. 1966 Dec 7;125(3):474–484. [PubMed] [Google Scholar]
- FRENCH J. E., MORRIS B. The tissue distribution and oxidation of 14C-labelled chylomicron fat injected intravenously in rats. J Physiol. 1958 Feb 17;140(2):262–271. doi: 10.1113/jphysiol.1958.sp005932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felts J. M., Berry M. N. The metabolism of free fatty acids and chylomicron triglyceride fatty acids by isolated rat liver cells. Biochim Biophys Acta. 1971 Feb 2;231(1):1–7. doi: 10.1016/0005-2760(71)90249-9. [DOI] [PubMed] [Google Scholar]
- Felts J. M., Itakura H., Crane R. T. The mechanism of assimilation of constituents of chylomicrons, very low density lipoproteins and remnants - a new theory. Biochem Biophys Res Commun. 1975 Oct 27;66(4):1467–1475. doi: 10.1016/0006-291x(75)90524-0. [DOI] [PubMed] [Google Scholar]
- Felts J. M., Mayes P. A. A computer programme for the calculation of radioactivity data from liquid-scintillation counters fitted with external standards. Biochem J. 1967 Nov;105(2):735–739. doi: 10.1042/bj1050735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felts J. M., Mayes P. A. Lack of uptake and oxidation of chylomicron triglyceride to carbon dioxide and ketone bodies by the perfused rat liver. Nature. 1965 Apr 10;206(980):195–196. doi: 10.1038/206195b0. [DOI] [PubMed] [Google Scholar]
- Felts J. M., Mayes P. A. Release of lipoprotein lipase from the perfused liver of the rat. Nature. 1967 May 6;214(5088):620–621. doi: 10.1038/214620a0. [DOI] [PubMed] [Google Scholar]
- Felts J. M. The metabolism of chylomicron triglyceride fatty acids by perfused rat livers and by intact rats. Ann N Y Acad Sci. 1965 Oct 8;131(1):24–33. doi: 10.1111/j.1749-6632.1965.tb34776.x. [DOI] [PubMed] [Google Scholar]
- Gardner R. S., Mayes P. A. Comparison of metabolism of the main lipid constituents of chylomicrons and chylomicron remnants in the perfused liver. Biochem Soc Trans. 1976;4(4):715–716. doi: 10.1042/bst0040715. [DOI] [PubMed] [Google Scholar]
- Kompiang I. P., Bensadoun A., Yang M. W. Effect of an anti-lipoprotein lipase serum on plasma triglyceride removal. J Lipid Res. 1976 Sep;17(5):498–505. [PubMed] [Google Scholar]
- MORRIS B. THE METABOLISM OF FREE FATTY ACIDS AND CHYLOMICRON TRIGLYCERIDES BY THE ISOLATED PERFUSED LIVER OF THE RAT. J Physiol. 1963 Oct;168:564–583. doi: 10.1113/jphysiol.1963.sp007208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes P. A., Felts J. M. Effect of haematocrit value and pO2 on the redox state and metabolism of the perfused liver. Biochem J. 1976 Jun 15;156(3):685–689. doi: 10.1042/bj1560685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes P. A., Felts J. M. The functional status of lipoprotein lipase in rat liver. Biochem J. 1968 Jul;108(3):483–487. doi: 10.1042/bj1080483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mjos O. D., Faergeman O., Hamilton R. L., Havel R. J. Characterization of remnants produced during the metabolism of triglyceride-rich lipoproteins of blood plasma and intestinal lymph in the rat. J Clin Invest. 1975 Sep;56(3):603–615. doi: 10.1172/JCI108130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NESTEL P. J., STEINBERG D. FATE OF PALMITATE AND OF LINOLEATE PERFUSED THROUGH THE ISOLATED RAT LIVER AT HIGH CONCENTRATIONS. J Lipid Res. 1963 Oct;4:461–469. [PubMed] [Google Scholar]
- Noel S. P., Dolphin P. J., Rubinstein D. An in vitro model for the catabolism of rat chylomicrons. Biochem Biophys Res Commun. 1975 Apr 7;63(3):764–772. doi: 10.1016/s0006-291x(75)80449-9. [DOI] [PubMed] [Google Scholar]
- RODBELL M., SCOW R. O., CHERNICK S. S. REMOVAL AND METABOLISM OF TRIGLYCERIDES BY PERFUSED LIVER. J Biol Chem. 1964 Feb;239:385–391. [PubMed] [Google Scholar]
- Raheja R. K., Kaur C., Singh A., Bhatia I. S. New colorimetric method for the quantitative estimation of phospholipids without acid digestion. J Lipid Res. 1973 Nov;14(6):695–697. [PubMed] [Google Scholar]
- Redgrave T. G. Formation of cholesteryl ester-rich particulate lipid during metabolism of chylomicrons. J Clin Invest. 1970 Mar;49(3):465–471. doi: 10.1172/JCI106255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle M. C., Smuckler E. A., Glomset J. A. Cholesteryl ester hydrolytic acitivity of rat liver plasma membrane. Biochim Biophys Acta. 1975 Jun 23;388(3):339–348. doi: 10.1016/0005-2760(75)90092-2. [DOI] [PubMed] [Google Scholar]
- Rudel L. L., Lee J. A., Morris M. D., Felts J. M. Characterization of plasma lipoproteins separated and purified by agarose-column chromatography. Biochem J. 1974 Apr;139(1):89–95. doi: 10.1042/bj1390089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudel L. L., Morris M. D. Determination of cholesterol using o-phthalaldehyde. J Lipid Res. 1973 May;14(3):364–366. [PubMed] [Google Scholar]
- Stein O., Stein Y. The role of the liver in the metabolism of chylomicrons, studied by electron microscopic autoradiography. Lab Invest. 1967 Oct;17(4):436–446. [PubMed] [Google Scholar]
- Topping D. L., Mayes P. A. Comparative effects of fructose and glucose on the lipid and carbohydrate metabolism of perfused rat liver. Br J Nutr. 1976 Jul;36(1):113–126. doi: 10.1079/bjn19760062. [DOI] [PubMed] [Google Scholar]
- Topping D. L., Mayes P. A. The immediate effects of insulin and fructose on the metabolism of the perfused liver. Changes in lipoprotein secretion, fatty acid oxidation and esterification, lipogenesis and carbohydrate metabolism. Biochem J. 1972 Jan;126(2):295–311. doi: 10.1042/bj1260295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waite M., Sisson P. Utilization of neutral glycerides and phosphatidylethanolamine by the phospholipase A-1 of the plasma membranes of rat liver. J Biol Chem. 1973 Dec 10;248(23):7985–7992. [PubMed] [Google Scholar]
- Waite M., Sisson P. Utilization of serum lipoprotein lipids by the monoacylglycerol acyltransferase. Biochim Biophys Acta. 1976 Dec 20;450(3):301–310. doi: 10.1016/0005-2760(76)90003-5. [DOI] [PubMed] [Google Scholar]


