Abstract
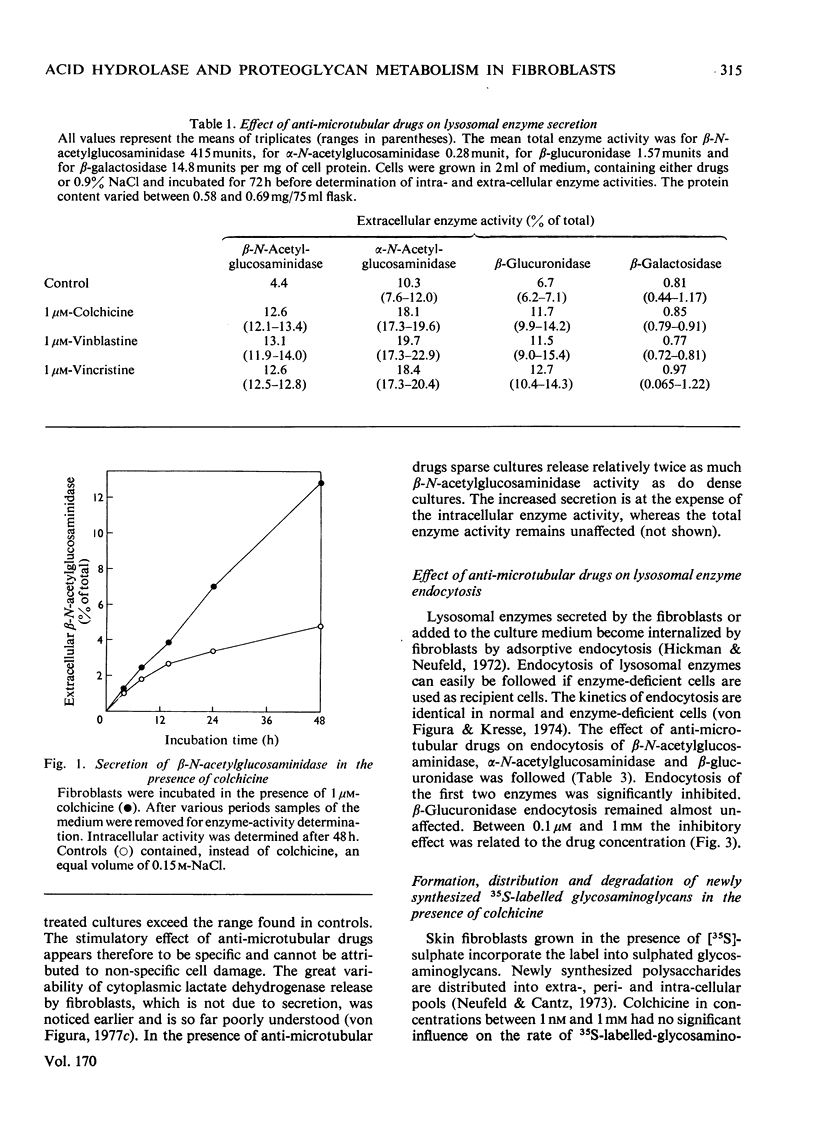
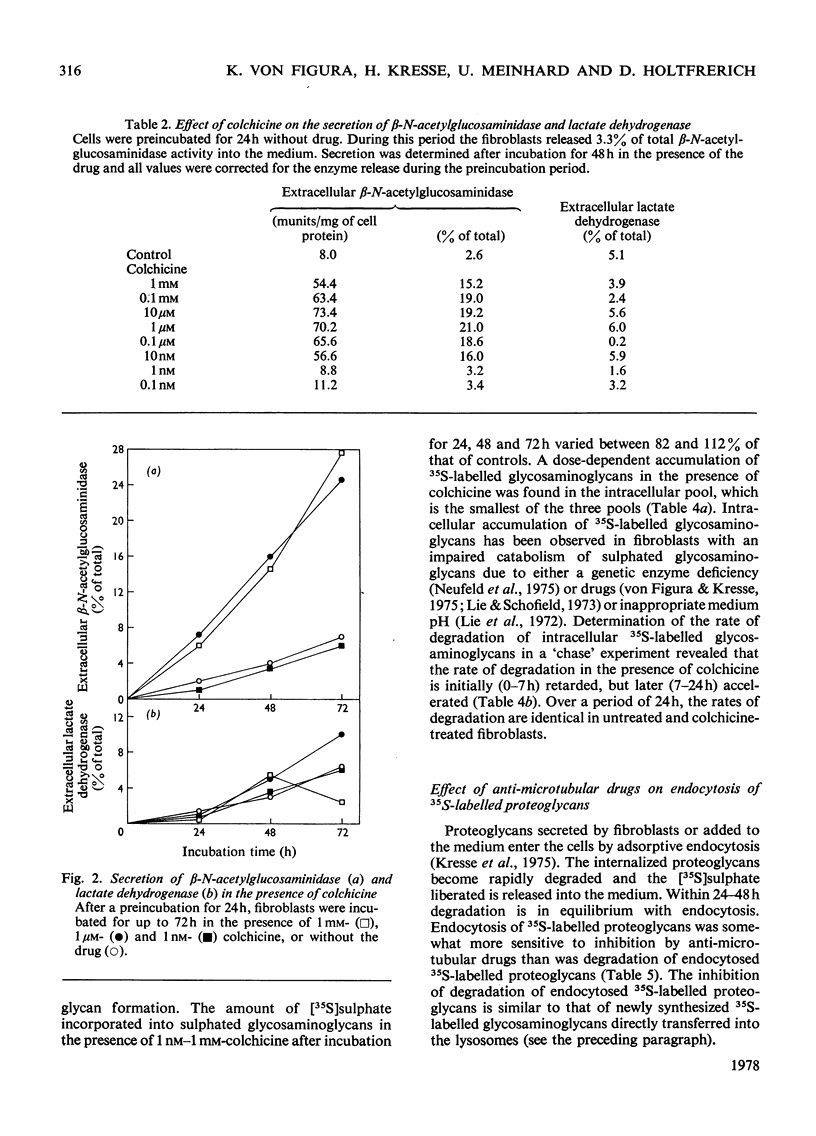
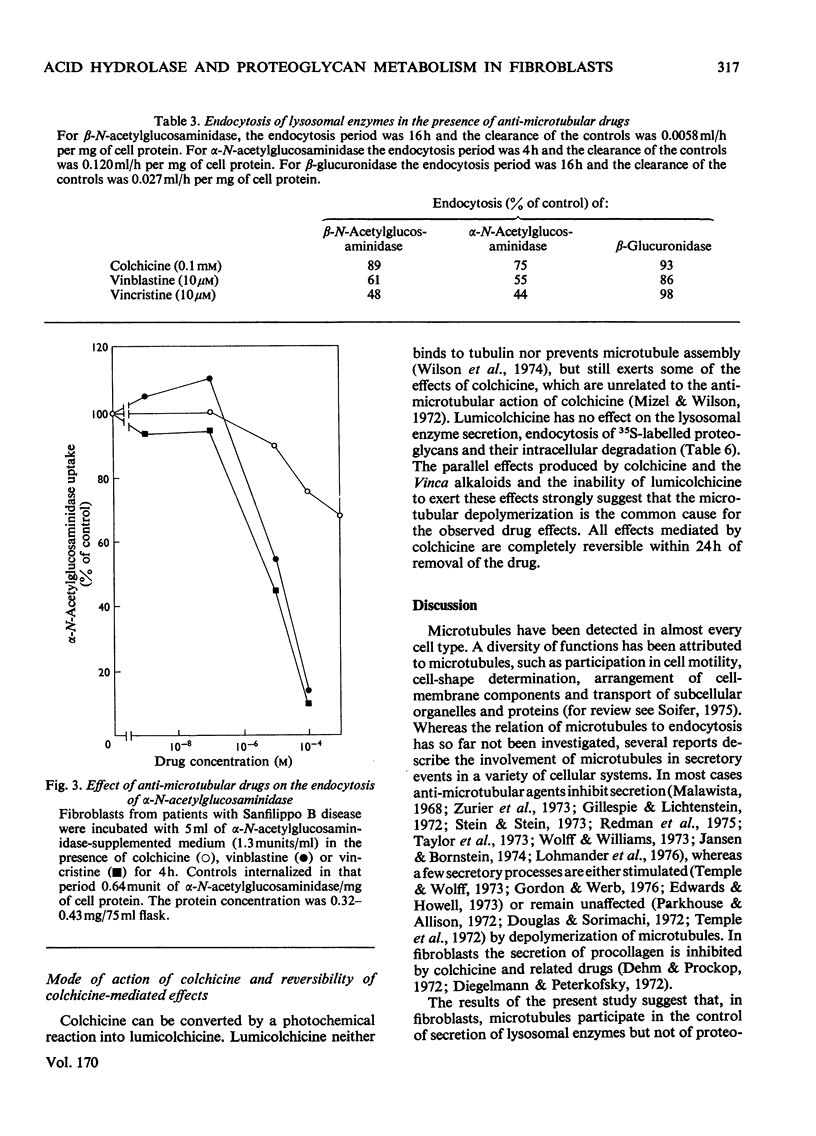
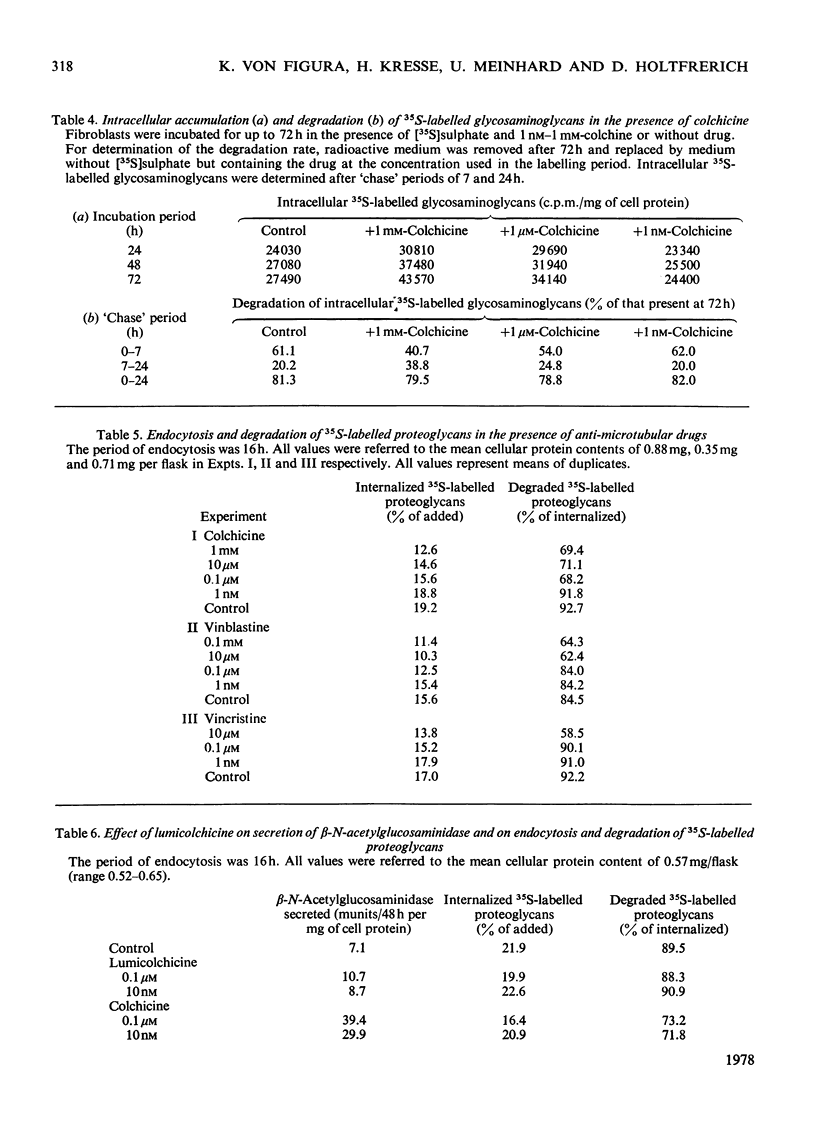
Fibroblasts were incubated in the presence of the anti-microtubular drugs colchicine, vinblastine and vincristine. In concentrations between 10nm and 1 mM these drugs stimulated the secretion of beta-N-acetylglucosaminidase, alpha-N-acetylglucosaminidase and beta-glucuronidase, but not of beta-galactosidase. The endocytosis of beta-N-acetylhexosaminidase and alpha-N-acetylglucosaminidase, but not of beta-glucuronidase, was inhibited at drug concentrations higher than 0.1 micrometer. Formation, secretion and association with the cell membrane of sulphated proteoglycans were not affected by anti-microtubular drugs. Endocytosis of sulphated proteoglycans and their subsequent degradation was inhibited by drug concentrations above 0.1 micrometer. The inhibition of intracellular glycosaminoglycan degradation led to a moderate storage of these compounds. These results suggest that microtubules participate in the control of secretion and endocytosis of lysosomal enzymes, and in the endocytosis and degradation of lysosomal substrates such as sulphated proteoglycans.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhattacharyya B., Volff J. Membrane-bound tubulin in brain and thyroid tissue. J Biol Chem. 1975 Oct 10;250(19):7639–7646. [PubMed] [Google Scholar]
- Bhattacharyya B., Wolff J. Polymerisation of membrane tubulin. Nature. 1976 Dec 9;264(5586):576–577. doi: 10.1038/264576a0. [DOI] [PubMed] [Google Scholar]
- Dehm P., Prockop D. J. Time lag in the secretion of collagen by matrix-free tendon cells and inhibition of the secretory process by colchicine and vinblastine. Biochim Biophys Acta. 1972 Apr 21;264(2):375–382. doi: 10.1016/0304-4165(72)90302-9. [DOI] [PubMed] [Google Scholar]
- Diegelmann R. F., Peterkofsky B. Inhibition of collagen secretion from bone and cultured fibroblasts by microtubular disruptive drugs. Proc Natl Acad Sci U S A. 1972 Apr;69(4):892–896. doi: 10.1073/pnas.69.4.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Sorimachi M. Colchicine inhibits adrenal medullary secretion evoked by acetylcholine without affecting that evoked by potassium. Br J Pharmacol. 1972 May;45(1):129–132. [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M. Surface modulation in cell recognition and cell growth. Science. 1976 Apr 16;192(4236):218–226. doi: 10.1126/science.769162. [DOI] [PubMed] [Google Scholar]
- Edwards J. C., Howell S. L. Effects of vinblastine and colchicine on the secretion of glucagon from isolated guinea-pig islets of langerhans. FEBS Lett. 1973 Feb 15;30(1):89–92. doi: 10.1016/0014-5793(73)80625-8. [DOI] [PubMed] [Google Scholar]
- Franke W. W. Cross-bridges between intramacronuclear microtubules and inner nuclear membrane. Z Naturforsch B. 1971 Jun;26(6):626–626. doi: 10.1515/znb-1971-0633. [DOI] [PubMed] [Google Scholar]
- Fratantoni J. C., Hall C. W., Neufeld E. F. The defect in Hurler's and Hunter's syndromes: faulty degradation of mucopolysaccharide. Proc Natl Acad Sci U S A. 1968 Jun;60(2):699–706. doi: 10.1073/pnas.60.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie E., Lichtenstein L. M. Histamine release from human leukocytes: studies with deuterium oxide, colchicine, and cytochalasin B. J Clin Invest. 1972 Nov;51(11):2941–2947. doi: 10.1172/JCI107118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstone A., Koenig H. Physicochemical modifications of lysosomal hydrolases during intracellular transport. Biochem J. 1973 Feb;132(2):267–282. doi: 10.1042/bj1320267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S., Werb Z. Secretion of macrophage neutral proteinase is enhanced by colchicine. Proc Natl Acad Sci U S A. 1976 Mar;73(3):872–876. doi: 10.1073/pnas.73.3.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman S., Neufeld E. F. A hypothesis for I-cell disease: defective hydrolases that do not enter lysosomes. Biochem Biophys Res Commun. 1972 Nov 15;49(4):992–999. doi: 10.1016/0006-291x(72)90310-5. [DOI] [PubMed] [Google Scholar]
- Jansen H. W., Bornstein P. Effects of antimicrotubular agents on glycosaminoglycan synthesis and secretion by embryonic chick cartilage and chondrocytes. Biochim Biophys Acta. 1974 Aug 7;362(1):150–159. doi: 10.1016/0304-4165(74)90036-1. [DOI] [PubMed] [Google Scholar]
- KALTENBACH J. P., KALTENBACH M. H., LYONS W. B. Nigrosin as a dye for differentiating live and dead ascites cells. Exp Cell Res. 1958 Aug;15(1):112–117. doi: 10.1016/0014-4827(58)90067-3. [DOI] [PubMed] [Google Scholar]
- Kresse H., Tekolf W., von Figura K., Buddecke E. Metabolism of sulfated glycosaminoglycans in cultivated bovine arterial cells. II. Quantitative studies on the uptake of 35SO4-labeled proteoglycans. Hoppe Seylers Z Physiol Chem. 1975 Jun;356(6):943–952. doi: 10.1515/bchm2.1975.356.s1.943. [DOI] [PubMed] [Google Scholar]
- Lie S. O., McKusick V. A., Neufeld E. F. Simulation of genetic mucopolysaccharidoses in normal human fibroblasts by alteration of pH of the medium. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2361–2363. doi: 10.1073/pnas.69.9.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie S. O., Schofield B. Inactivation of lysosomal function in normal cultured human fibroblasts by chloroquine. Biochem Pharmacol. 1973 Dec 1;22(23):3109–3114. doi: 10.1016/0006-2952(73)90197-4. [DOI] [PubMed] [Google Scholar]
- Lohmander S., Moskalewski S., Madsen K., Thyberg J., Friberg U. Influence of colchicine on the synthesis and secretion of proteoglycans and collagen by fetal guinea pig chondrocytes. Exp Cell Res. 1976 May;99(2):333–345. doi: 10.1016/0014-4827(76)90591-7. [DOI] [PubMed] [Google Scholar]
- Malawista S. E. Colchicine: a common mechanism for its anti-inflammatory and anti-mitotic effects. Arthritis Rheum. 1968 Apr;11(2):191–197. doi: 10.1002/art.1780110210. [DOI] [PubMed] [Google Scholar]
- McClain D. A., D'Eustachio P., Edelman G. M. Role of surface modulating assemblies in growth control of normal and transformed fibroblasts. Proc Natl Acad Sci U S A. 1977 Feb;74(2):666–670. doi: 10.1073/pnas.74.2.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizel S. B., Wilson L. Nucleoside transport in mammalian cells. Inhibition by colchicine. Biochemistry. 1972 Jul 4;11(14):2573–2578. doi: 10.1021/bi00764a003. [DOI] [PubMed] [Google Scholar]
- Neufeld E. F., Lim T. W., Shapiro L. J. Inherited disorders of lysosomal metabolism. Annu Rev Biochem. 1975;44:357–376. doi: 10.1146/annurev.bi.44.070175.002041. [DOI] [PubMed] [Google Scholar]
- Parkhouse R. M., Allison A. C. Failure of cytochalasin or colchicine to inhibit secretion of immunoglobulins. Nat New Biol. 1972 Feb 16;235(59):220–222. doi: 10.1038/newbio235220a0. [DOI] [PubMed] [Google Scholar]
- Redman C. M., Banerjee D., Howell K., Palade G. E. The step at which colchicine blocks the secretion of plasma protein by rat liver. Ann N Y Acad Sci. 1975 Jun 30;253:780–788. doi: 10.1111/j.1749-6632.1975.tb19246.x. [DOI] [PubMed] [Google Scholar]
- Stein O., Stein Y. Colchicine-induced inhibition of very low density lipoprotein release by rat liver in vivo. Biochim Biophys Acta. 1973 Apr 13;306(1):142–147. doi: 10.1016/0005-2760(73)90219-1. [DOI] [PubMed] [Google Scholar]
- Taylor A., Mamelak M., Reaven E., Maffly R. Vasopressin: possible role of microtubules and microfilaments in its action. Science. 1973 Jul 27;181(4097):347–350. doi: 10.1126/science.181.4097.347. [DOI] [PubMed] [Google Scholar]
- Temple R., Williams J. A., Wilber J. F., Wolff J. Colchicine and hormone secretion. Biochem Biophys Res Commun. 1972 Feb 16;46(3):1454–1461. doi: 10.1016/s0006-291x(72)80140-2. [DOI] [PubMed] [Google Scholar]
- Temple R., Wolff J. Stimulation of steroid secretion by antimicrotubular agents. J Biol Chem. 1973 Apr 25;248(8):2691–2698. [PubMed] [Google Scholar]
- Wessells N. K., Spooner B. S., Ash J. F., Bradley M. O., Luduena M. A., Taylor E. L., Wrenn J. T., Yamada K. Microfilaments in cellular and developmental processes. Science. 1971 Jan 15;171(3967):135–143. doi: 10.1126/science.171.3967.135. [DOI] [PubMed] [Google Scholar]
- Wilson L., Bamburg J. R., Mizel S. B., Grisham L. M., Creswell K. M. Interaction of drugs with microtubule proteins. Fed Proc. 1974 Feb;33(2):158–166. [PubMed] [Google Scholar]
- Wilson L., Friedkin M. The biochemical events of mitosis. I. Synthesis and properties of colchicine labeled with tritium in its acetyl moiety. Biochemistry. 1966 Jul;5(7):2463–2468. doi: 10.1021/bi00871a042. [DOI] [PubMed] [Google Scholar]
- Wolff J., Williams J. A. The role of microtubles and microfilaments in thyroid secretion. Recent Prog Horm Res. 1973;29:229–285. doi: 10.1016/b978-0-12-571129-6.50010-5. [DOI] [PubMed] [Google Scholar]
- Yahara I., Edelman G. M. Electron microscopic analysis of the modulation of lymphocyte receptor mobility. Exp Cell Res. 1975 Mar 1;91(1):125–142. doi: 10.1016/0014-4827(75)90150-0. [DOI] [PubMed] [Google Scholar]
- Zurier R. B., Hoffstein S., Weissmann G. Mechanisms of lysosomal enzyme release from human leukocytes. I. Effect of cyclic nucleotides and colchicine. J Cell Biol. 1973 Jul;58(1):27–41. doi: 10.1083/jcb.58.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurier R. B., Weissmann G., Hoffstein S., Kammerman S., Tai H. H. Mechanisms of lysosomal enzyme release from human leukocytes. II. Effects of cAMP and cGMP, autonomic agonists, and agents which affect microtubule function. J Clin Invest. 1974 Jan;53(1):297–309. doi: 10.1172/JCI107550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Figura K. Human alpha-N-acetylglucosaminidase. 1. Purification and properties. Eur J Biochem. 1977 Nov 1;80(2):523–533. [PubMed] [Google Scholar]
- von Figura K., Kresse H. Inhibition of pinocytosis by cytochalasin B. Decrease in intracellular lysosomal-enzyme activities and increased storage of glycosaminoglycans. Eur J Biochem. 1974 Oct 2;48(2):357–363. doi: 10.1111/j.1432-1033.1974.tb03777.x. [DOI] [PubMed] [Google Scholar]
- von Figura K., Kresse H. Quantitative aspects of pinocytosis and the intracellular fate of N-acetyl-alpha-D-glucosaminidase in Sanfilippo B fibroblasts. J Clin Invest. 1974 Jan;53(1):85–90. doi: 10.1172/JCI107563. [DOI] [PMC free article] [PubMed] [Google Scholar]


