Abstract
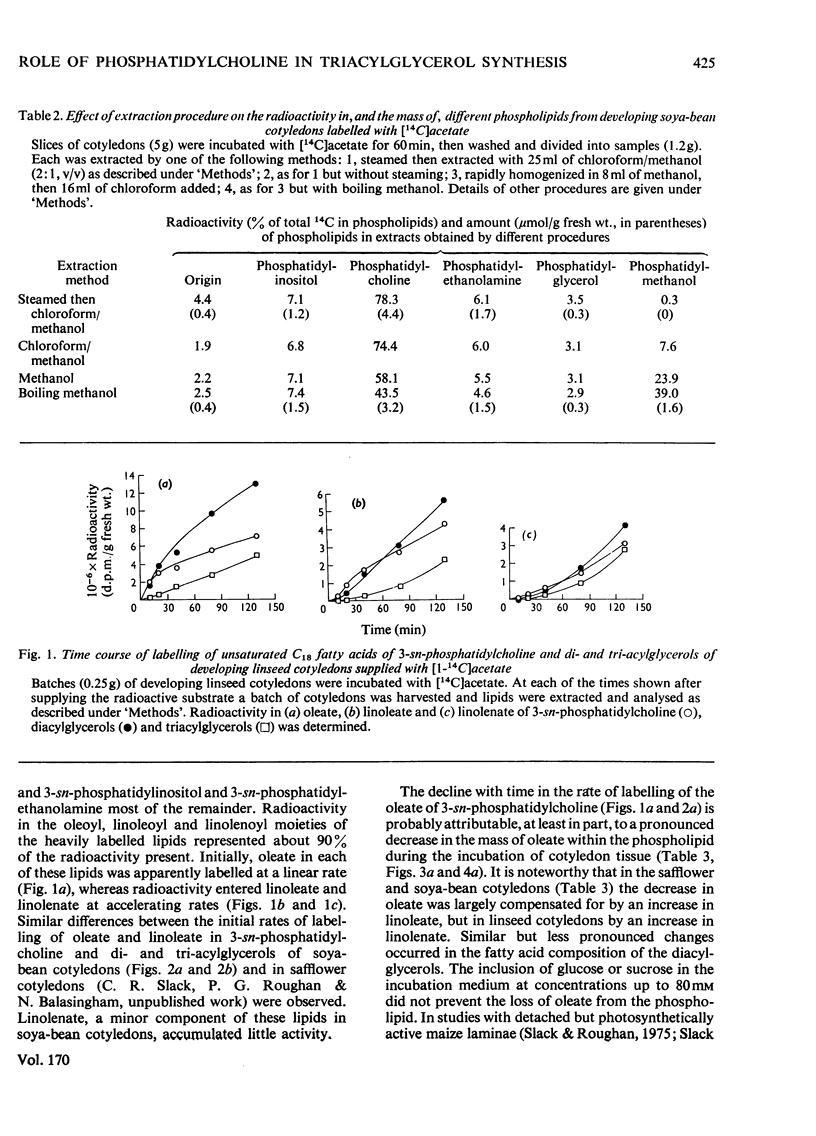
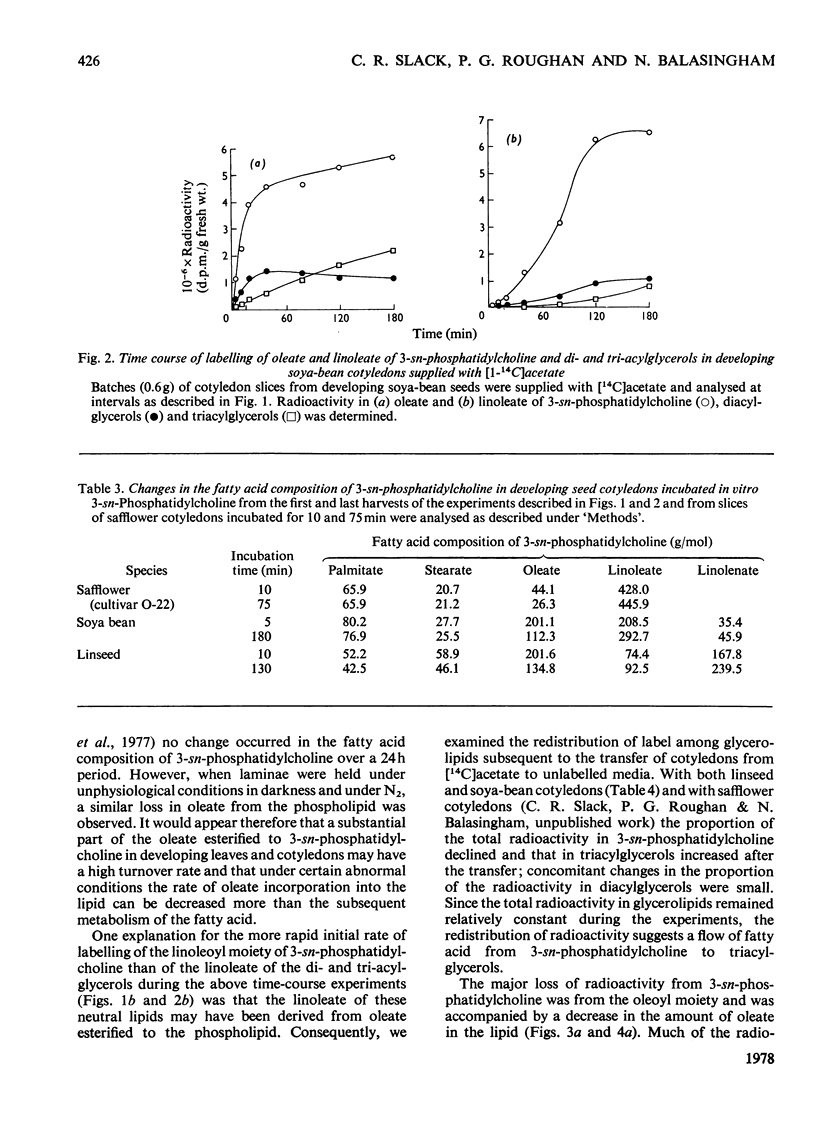
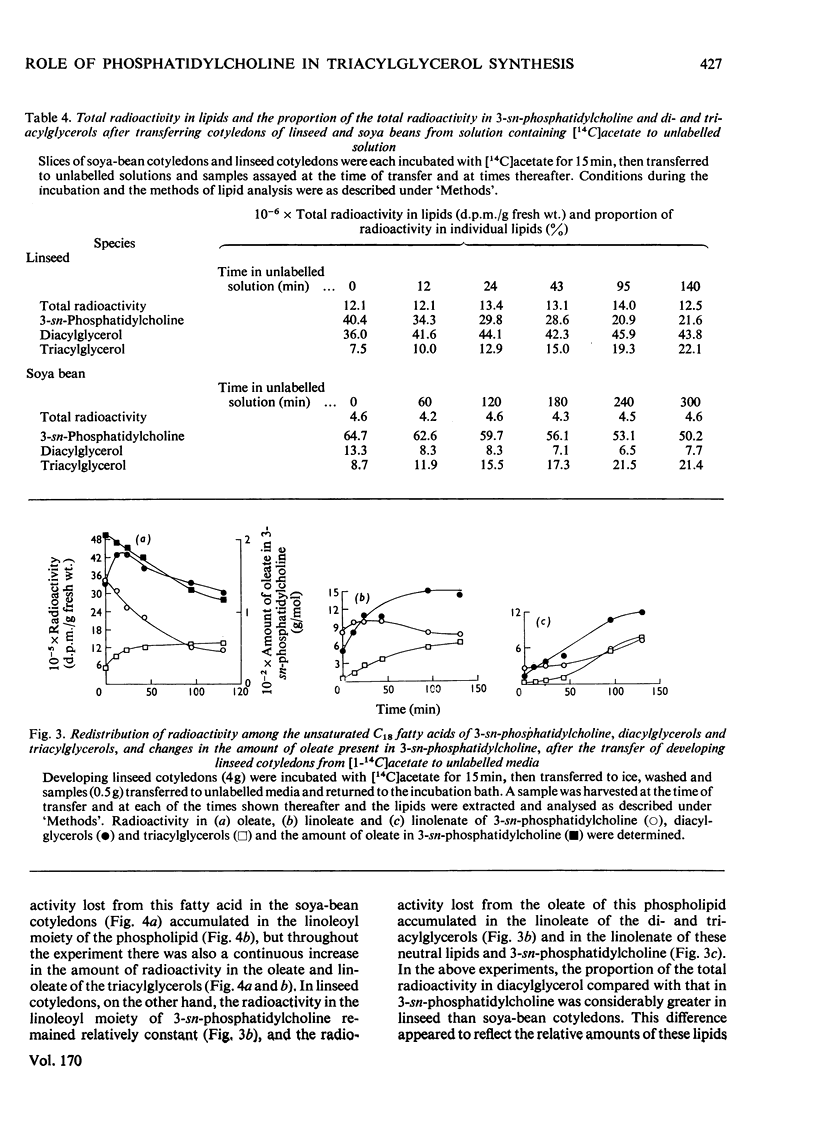
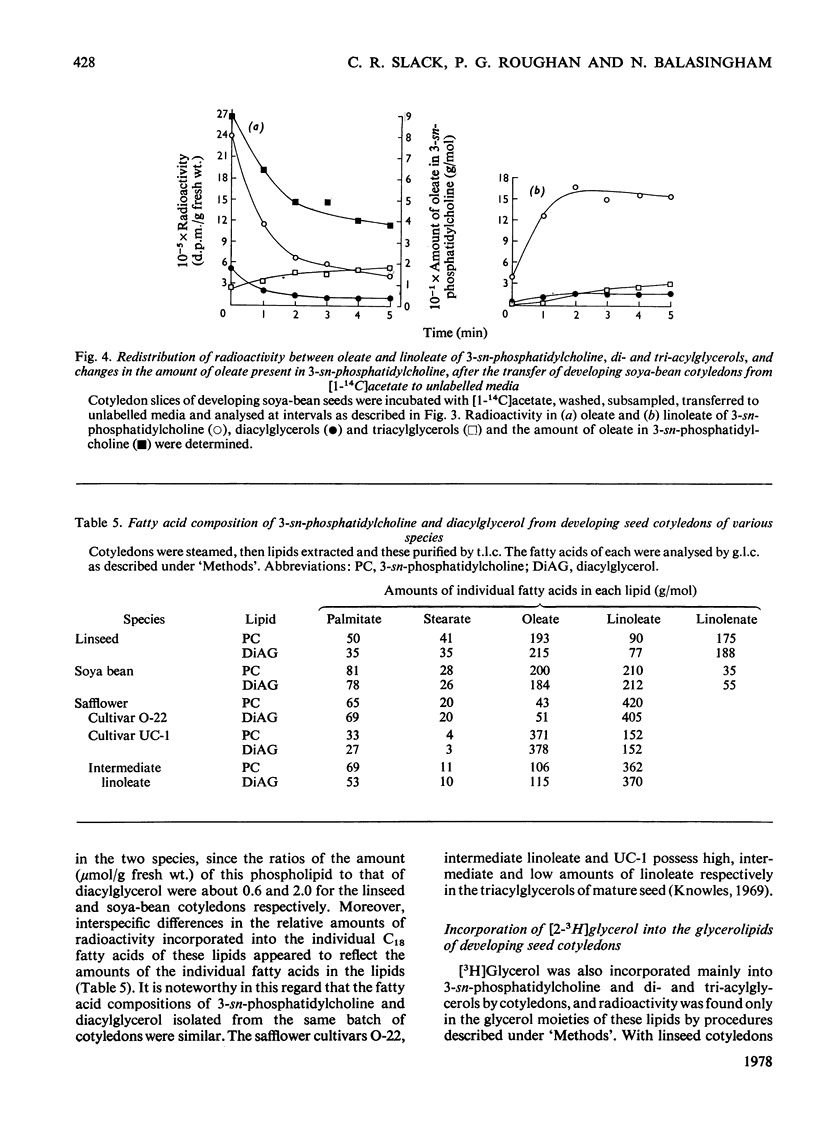
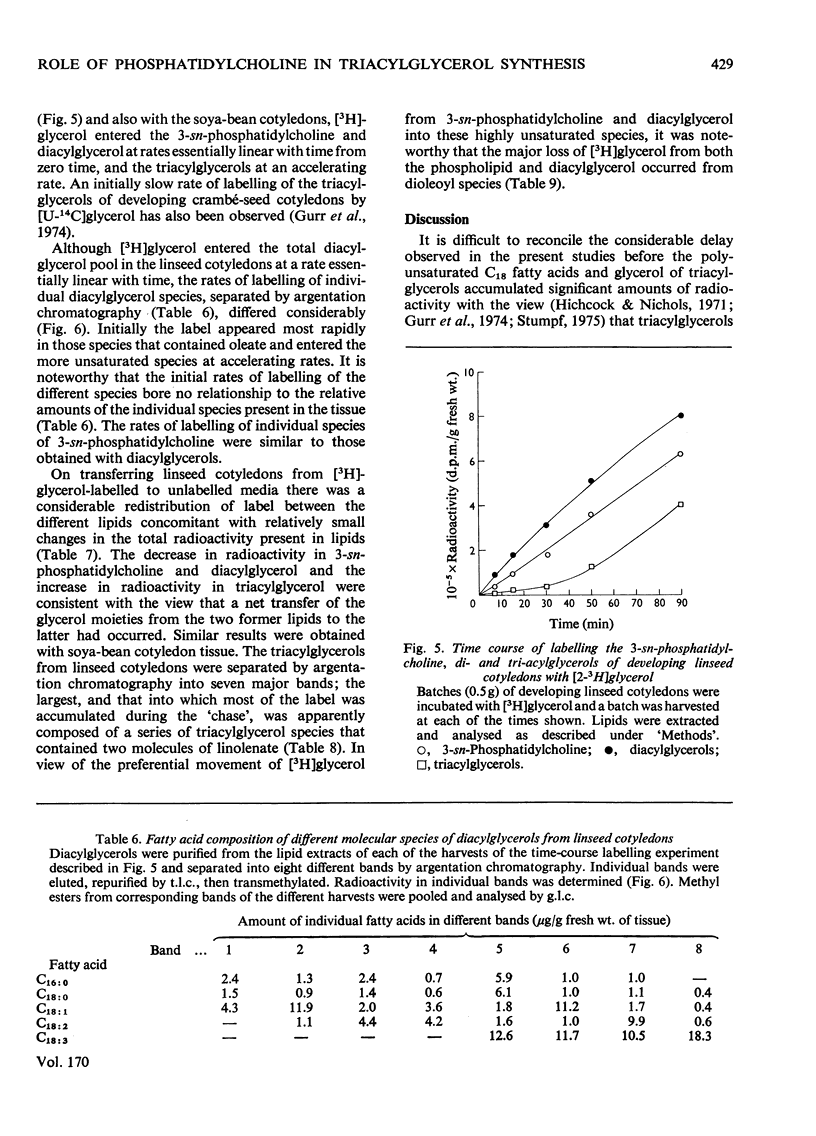
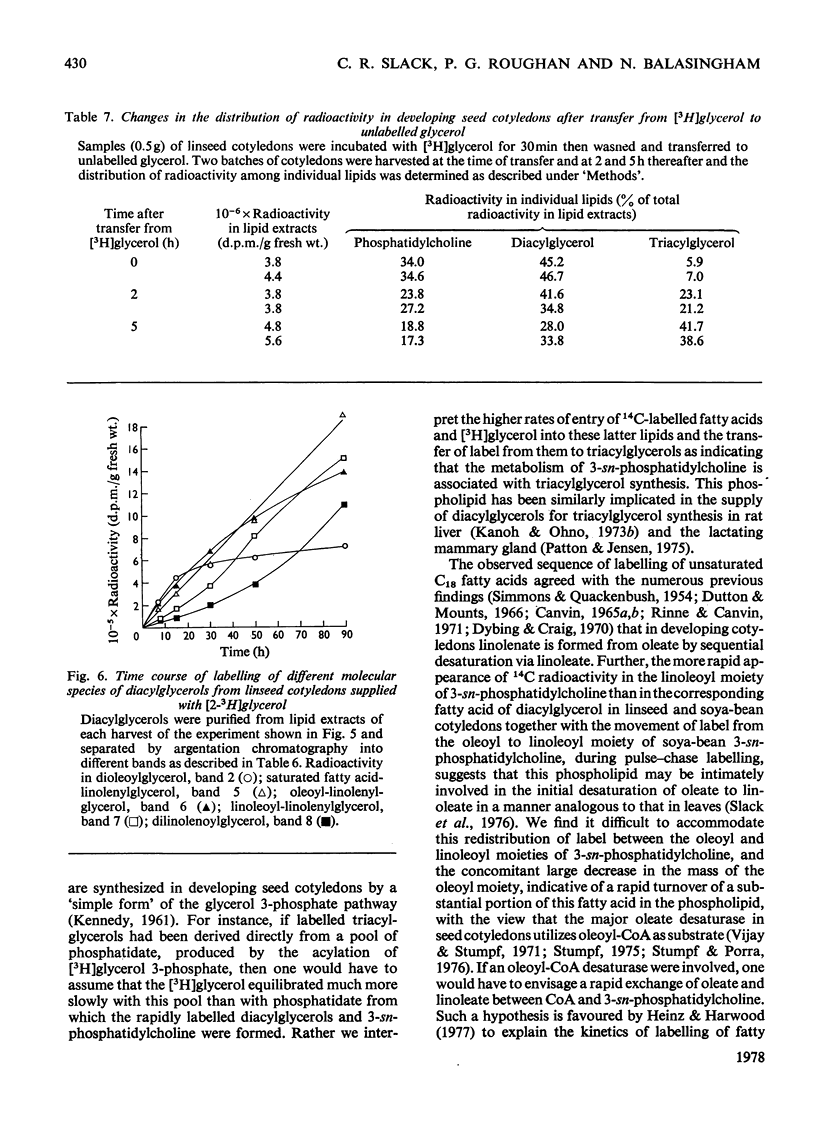
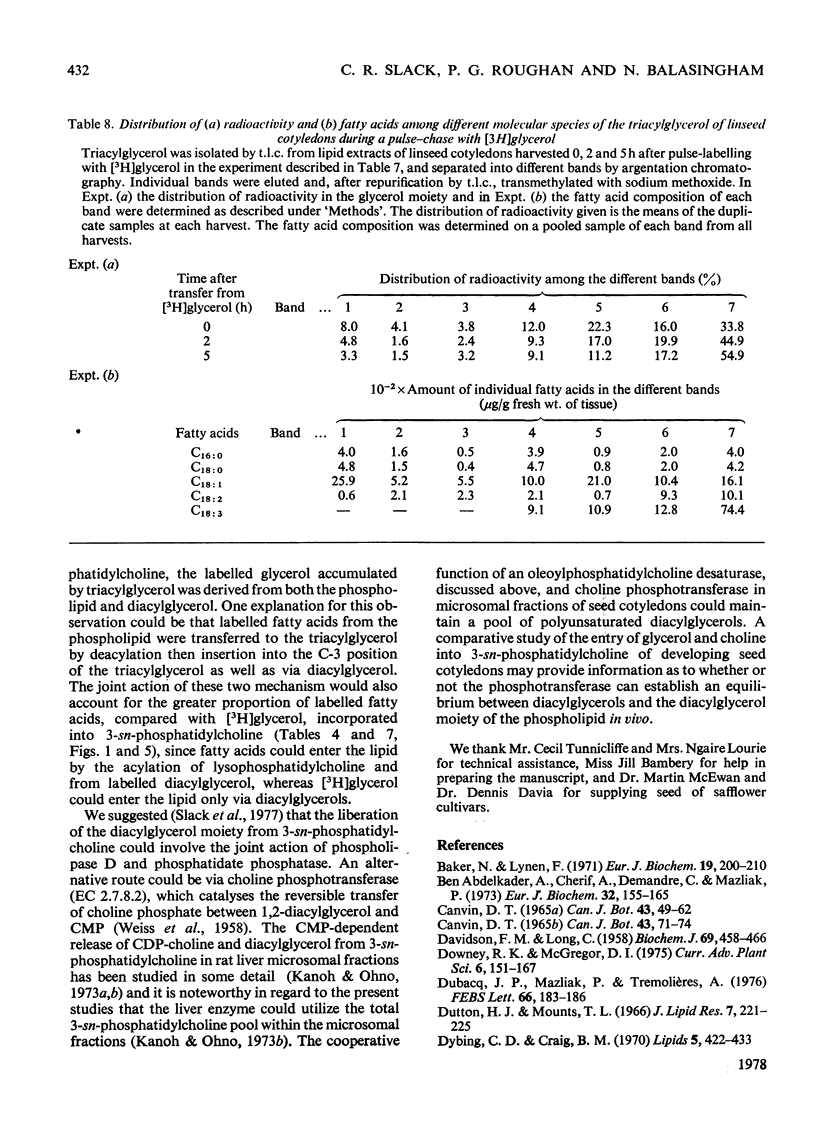
1. 3-sn-Phosphatidylcholine was identified as the major lipid in cotyledons from the developing seeds of soya bean, linseed and safflower when tissue was steamed before lipid extraction. The proportion of oleate in this lipid decreased markedly and that of the polyunsaturated C18 fatty acids increased when detached developing cotyledons were incubated for up to 3h. Similar but less pronounced changes occurred in diacylglycerol, which had a fatty acid composition resembling that of the 3-sn-phosphatidylcholine from cotyledons of the same species. 2. [1-14C]Acetate supplied to detached cotyledons was incorporated into the acyl moieties of mainly 3-sn-phosphatidylcholine, 1,2-diacylglycerol and triacylglycerol. Initially label was predominantly in oleate, but subsequently entered at accelerating rates the linoleoyl moieties of the above lipids in soya-bean and safflower cotyledons and the linoleoyl and linolenyl moieties of these lipids in linseed cotyledons. In pulse–chase experiments label was rapidly lost from the oleate of 3-sn-phosphatidylcholine and accumulated in the linoleoyl and linolenoyl moieties of this phospholipid and of the di- and tri-acylglycerols. 3. [2-3H]Glycerol was incorporated into the glycerol moieties of mainly 3-sn-phosphatidylcholine and di- and tri-acylglycerols of developing linseed and soya-bean cotyledons. The label entered the phospholipid and diacylglycerol at rates essentially linear with time from the moment the substrate was supplied, and entered the triacylglycerol at an accelerating rate. With linseed cotyledons the labelled glycerol was incorporated initially mainly into species of 3-sn-phosphatidylcholine and diacylglycerol that contained oleate, but accumulated with time in more highly unsaturated species. In pulse–chase experiments with linseed cotyledons, label was lost from both 3-sn-phosphatidylcholine and diacylglycerol, preferentially from the dioleoyl species, and accumulated in triacylglycerol, mainly in species containing two molecules of linolenate. 4. The results suggest a rapid turnover of 3-sn-phosphatidylcholine during triacylglycerol accumulation in developing oilseeds, and are consistent with the operation of a biosynthetic route whereby oleate initially esterified to the phospholipid is first desaturated, then polyunsaturated fatty acids transferred to triacylglycerol, via diacylglycerol. The possible role of oleoyl phosphatidylcholine as a substrate for oleate desaturation is discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdelkader A. B., Cherif A., Demandre C., Mazliak P. The oleyl-coenzyme-A desaturase of potato tubers. Enzymatic properties, intracellular localization and induction during "aging" of tuber slices. Eur J Biochem. 1973 Jan 3;32(1):155–165. doi: 10.1111/j.1432-1033.1973.tb02592.x. [DOI] [PubMed] [Google Scholar]
- Baker N., Lynen F. Factors involved in fatty acyl CoA desaturation by fungal microsomes. The relative roles of acyl CoA and phospholipids as substrates. Eur J Biochem. 1971 Mar 11;19(2):200–210. doi: 10.1111/j.1432-1033.1971.tb01305.x. [DOI] [PubMed] [Google Scholar]
- DAVIDSON F. M., LONG C. The structure of the naturally occurring phosphoglycerides. 4. Action of cabbage-leaf phospholipase D on ovolecithin and related substances. Biochem J. 1958 Jul;69(3):458–466. doi: 10.1042/bj0690458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton H. J., Mounts T. L. Desaturation of fatty acids in seeds of higher plants. J Lipid Res. 1966 Mar;7(2):221–225. [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fricke U. Tritosol: a new scintillation cocktail based on Triton X-100. Anal Biochem. 1975 Feb;63(2):555–558. doi: 10.1016/0003-2697(75)90379-6. [DOI] [PubMed] [Google Scholar]
- Gurr M. I., Blades J., Appleby R. S., Smith C. G., Robinson M. P., Nichols B. W. Studies on seed-oil triglycerides. Triglyceride biosynthesis and storage in whole seeds and oil bodies of Crambe abyssinica. Eur J Biochem. 1974 Apr 1;43(2):281–290. doi: 10.1111/j.1432-1033.1974.tb03411.x. [DOI] [PubMed] [Google Scholar]
- Gurr M. I., Blades J., Appleby R. S. Studies on seed-oil triglycerides. The composition of Crambé abyssinica triglycerides during seed maturation. Eur J Biochem. 1972 Sep 18;29(2):362–368. doi: 10.1111/j.1432-1033.1972.tb01997.x. [DOI] [PubMed] [Google Scholar]
- Gurr M. I., Brawn P. The biosynthesis of polyunsaturated fatty acids by photosynthetic tissue. The composition of phosphatidyl choline species in Chlorella vulgaris during the formation of linoleic acid. Eur J Biochem. 1970 Nov;17(1):19–22. doi: 10.1111/j.1432-1033.1970.tb01126.x. [DOI] [PubMed] [Google Scholar]
- Gurr M. I., Robinson M. P., James A. T. The mechanism of formation of polyunsaturated fatty acids by photosynthetic tissue. The tight coupling of oleate desaturation with phospholipid synthesis in Chlorella vulgaris. Eur J Biochem. 1969 May 1;9(1):70–78. doi: 10.1111/j.1432-1033.1969.tb00577.x. [DOI] [PubMed] [Google Scholar]
- Heinz E., Harwood J. L. Incorporation of carbon dioxide, acetate and sulphate into the glycerolipids of Vicia faba leaves. Hoppe Seylers Z Physiol Chem. 1977 Jul;358(7):897–908. doi: 10.1515/bchm2.1977.358.2.897. [DOI] [PubMed] [Google Scholar]
- Imokawa G., Sumura K., Katsumi M. Study on skin roughness caused by surfactants: II. Correlation between protein denaturation and skin roughness. J Am Oil Chem Soc. 1975 Dec;52(12):484–489. doi: 10.1007/BF02640737. [DOI] [PubMed] [Google Scholar]
- KENNEDY E. P. Biosynthesis of complex lipids. Fed Proc. 1961 Dec;20:934–940. [PubMed] [Google Scholar]
- Kader J. C. Cyanide sensitivity and induction of the microsomal oleoyl-CoA desaturase of potato tuber. Biochim Biophys Acta. 1977 Mar 25;486(3):429–436. doi: 10.1016/0005-2760(77)90092-3. [DOI] [PubMed] [Google Scholar]
- Kano H., Ono K. Studies on 1,2-diglycerides formed from endogenous lecithins by the back-reaction of rat liver microsomal CDPcholine: 1,2-diacylglycerol cholinephosphotransferase. Biochim Biophys Acta. 1973 Oct 17;326(1):17–25. [PubMed] [Google Scholar]
- Kano H., Ono K. Utilization of endogenous phospholipids by the backreaction of CDP-choline (-ethanolamine): 1,2-diglyceride choline (ethanolamine)-phosphotransferase in rat liver microsomes. Biochim Biophys Acta. 1973 May 24;306(2):203–217. [PubMed] [Google Scholar]
- Long C., Odavić R., Sargent E. J. The action of cabbage-leaf phospholipase D upon lysolecithin. Biochem J. 1967 Jan;102(1):216–220. doi: 10.1042/bj1020216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris L. J. Separations of lipids by silver ion chromatography. J Lipid Res. 1966 Nov;7(6):717–732. [PubMed] [Google Scholar]
- Nagai J., Bloch K. Enzymatic desaturation of stearyl acyl carrier protein. J Biol Chem. 1968 Sep 10;243(17):4626–4633. [PubMed] [Google Scholar]
- Privett O. S., Dougherty K. A., Erdahl W. L., Stolyhwo A. Studies on the lipid composition of developing soybeans. J Am Oil Chem Soc. 1973 Dec;50(12):516–520. doi: 10.1007/BF02640523. [DOI] [PubMed] [Google Scholar]
- Pugh E. L., Kates M. Characterization of a membrane-bound phospholipid desaturase system of candida lipolytica. Biochim Biophys Acta. 1975 Mar 24;380(3):442–453. doi: 10.1016/0005-2760(75)90112-5. [DOI] [PubMed] [Google Scholar]
- Pugh E. L., Kates M. Desaturation of phosphatidylcholine and phosphatidylethanolamine by a microsomal enzyme system from Candida lipolytica. Biochim Biophys Acta. 1973 Sep 25;316(3):305–316. doi: 10.1016/0005-2760(73)90071-4. [DOI] [PubMed] [Google Scholar]
- Roughan P. G., Boardman N. K. Lipid Composition of Pea and Bean Leaves during Chloroplast Development. Plant Physiol. 1972 Jul;50(1):31–34. doi: 10.1104/pp.50.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouser G., Siakotos A. N., Fleischer S. Quantitative analysis of phospholipids by thin-layer chromatography and phosphorus analysis of spots. Lipids. 1966 Jan;1(1):85–86. doi: 10.1007/BF02668129. [DOI] [PubMed] [Google Scholar]
- Shine W. E., Mancha M., Stumpf P. K. Fat metabolism in higher plants. Differential incorporation of acyl-coenzymes A and acyl-acyl carrier proteins into plant microsomal lipids. Arch Biochem Biophys. 1976 Apr;173(2):472–479. doi: 10.1016/0003-9861(76)90284-8. [DOI] [PubMed] [Google Scholar]
- Singh H., Privett O. S. Incorporation of 33P in soybean phosphatides. Biochim Biophys Acta. 1970 Feb 10;202(1):200–202. doi: 10.1016/0005-2760(70)90236-5. [DOI] [PubMed] [Google Scholar]
- Slack C. R., Roughan P. G., Balasingham N. Labelling studies in vivo on the metabolism of the acyl and glycerol moieties of the glycerolipids in the developing maize leaf. Biochem J. 1977 Feb 15;162(2):289–296. doi: 10.1042/bj1620289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack C. R., Roughan P. G., Terpstra J. Some properties of a microsomal oleate desaturase from leaves. Biochem J. 1976 Apr 1;155(1):71–80. doi: 10.1042/bj1550071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack C. R., Roughan P. G. The kinetics of incorporation in vivo of (14C)acetate and (14C)carbon dioxide into the fatty acids of glycerolipids in developing leaves. Biochem J. 1975 Nov;152(2):217–228. doi: 10.1042/bj1520217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpf P. K., Porra R. J. Lipid biosynthesis in developing and germinating soybean cotyledons. The formation of oleate by a soluble stearyl acyl carrier protein desaturase. Arch Biochem Biophys. 1976 Sep;176(1):63–70. doi: 10.1016/0003-9861(76)90141-7. [DOI] [PubMed] [Google Scholar]
- Talamo B., Chang N., Bloch K. Desaturation of oleyl phospholipid to linoleyl phospholipid in Torulopsis utilis. J Biol Chem. 1973 Apr 25;248(8):2738–2742. [PubMed] [Google Scholar]
- Vijay I. K., Stumpf P. K. Fat metabolism in higher plants. XLVI. Nature of the substrate and the product of oleyl coenzyme A desaturase from Carthamus tinctorius. J Biol Chem. 1971 May 10;246(9):2910–2917. [PubMed] [Google Scholar]
- WEISS S. B., SMITH S. W., KENNEDY E. P. The enzymatic formation of lecithin from cytidine diphosphate choline and D-1,2-diglyceride. J Biol Chem. 1958 Mar;231(1):53–64. [PubMed] [Google Scholar]
- Wilson R. F., Rinne R. W. Effect of freezing and cold storage on phospholipids in developing soybean cotyledons. Plant Physiol. 1976 Feb;57(2):270–273. doi: 10.1104/pp.57.2.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. F., Rinne R. W. Phospholipids in the developing soybean seed. Plant Physiol. 1974 Nov;54(5):744–747. doi: 10.1104/pp.54.5.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. F., Rinne R. W. Studies on lipid synthesis and degradation in developing soybean cotyledons. Plant Physiol. 1976 Mar;57(3):375–381. doi: 10.1104/pp.57.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]


