Abstract
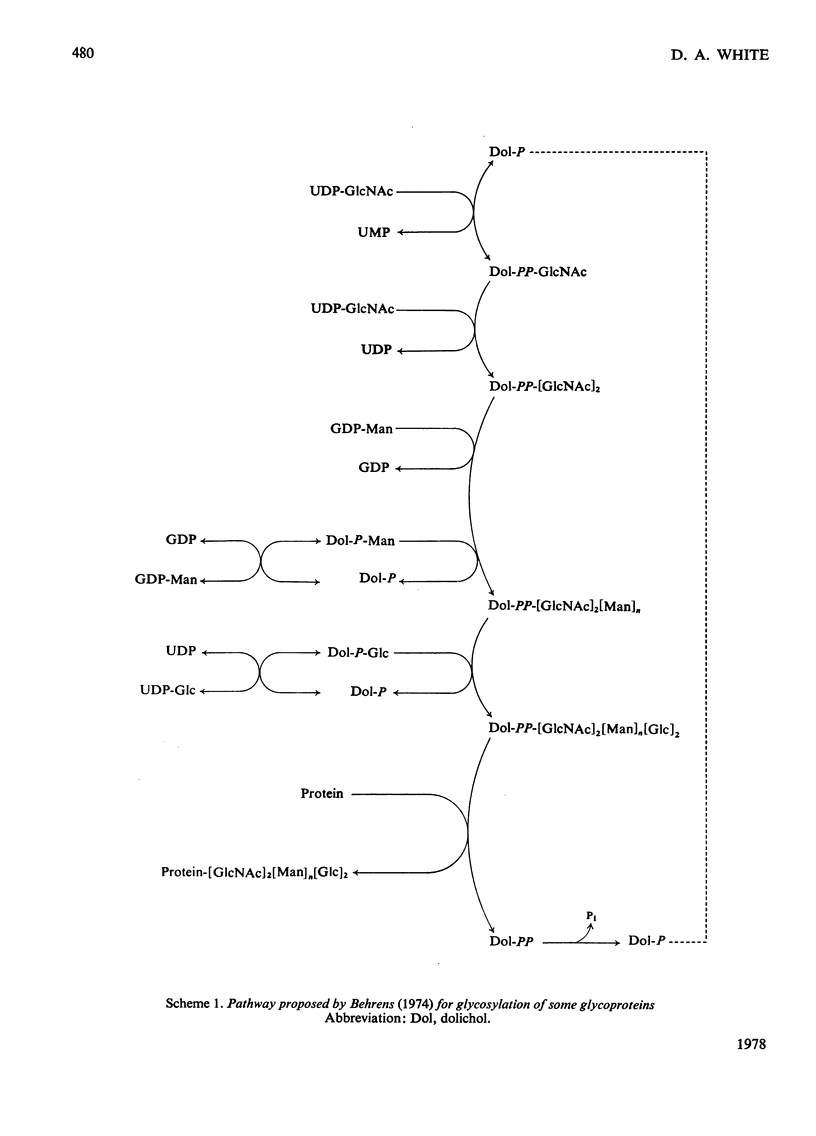
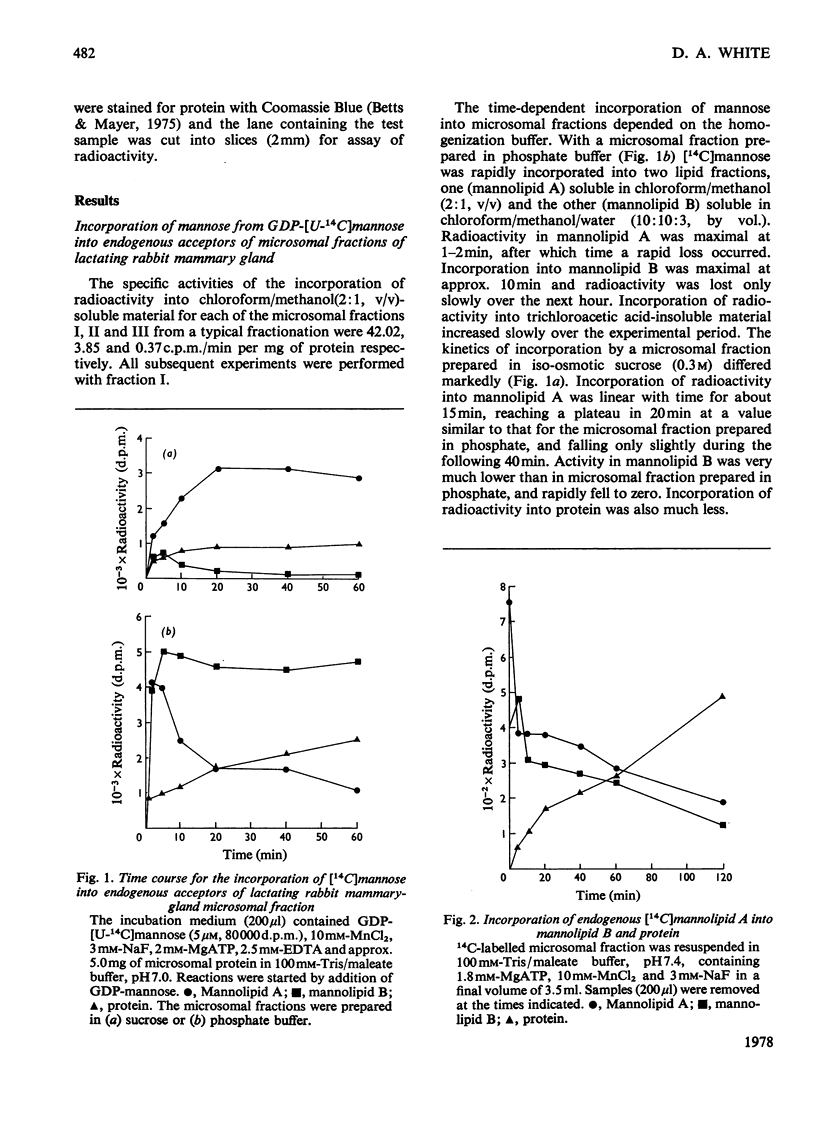
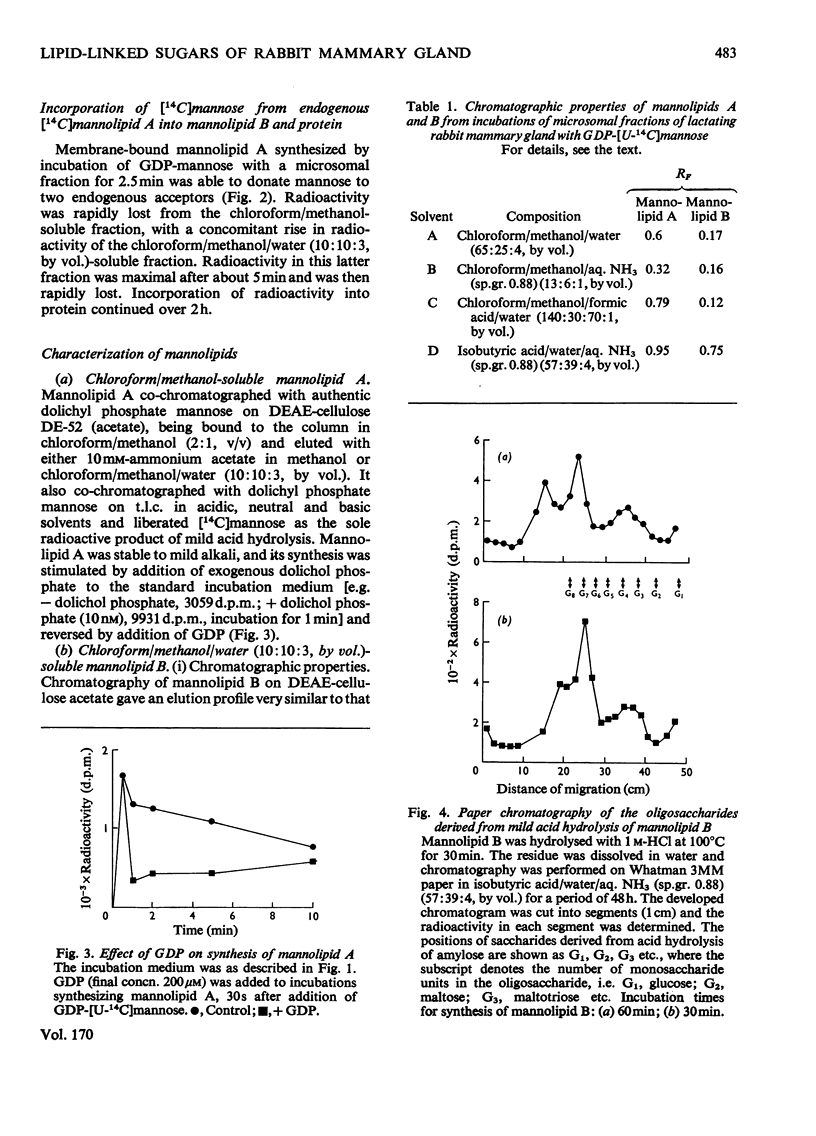
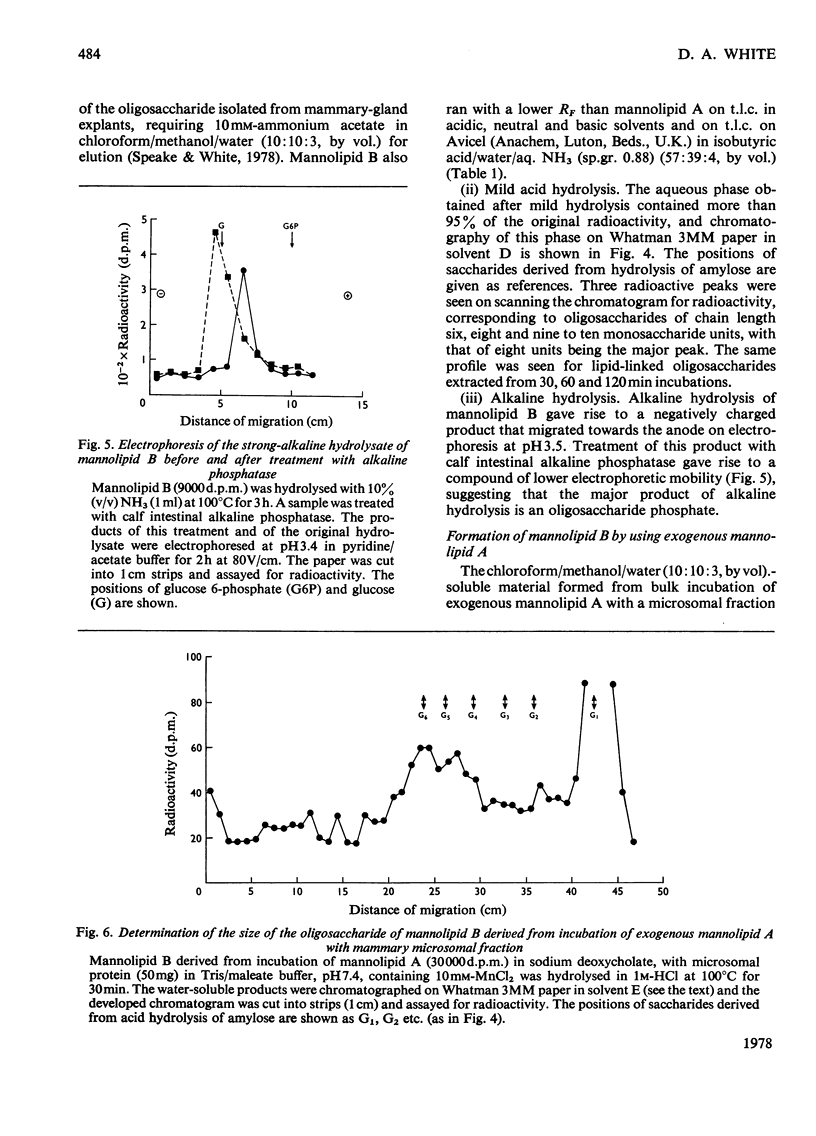
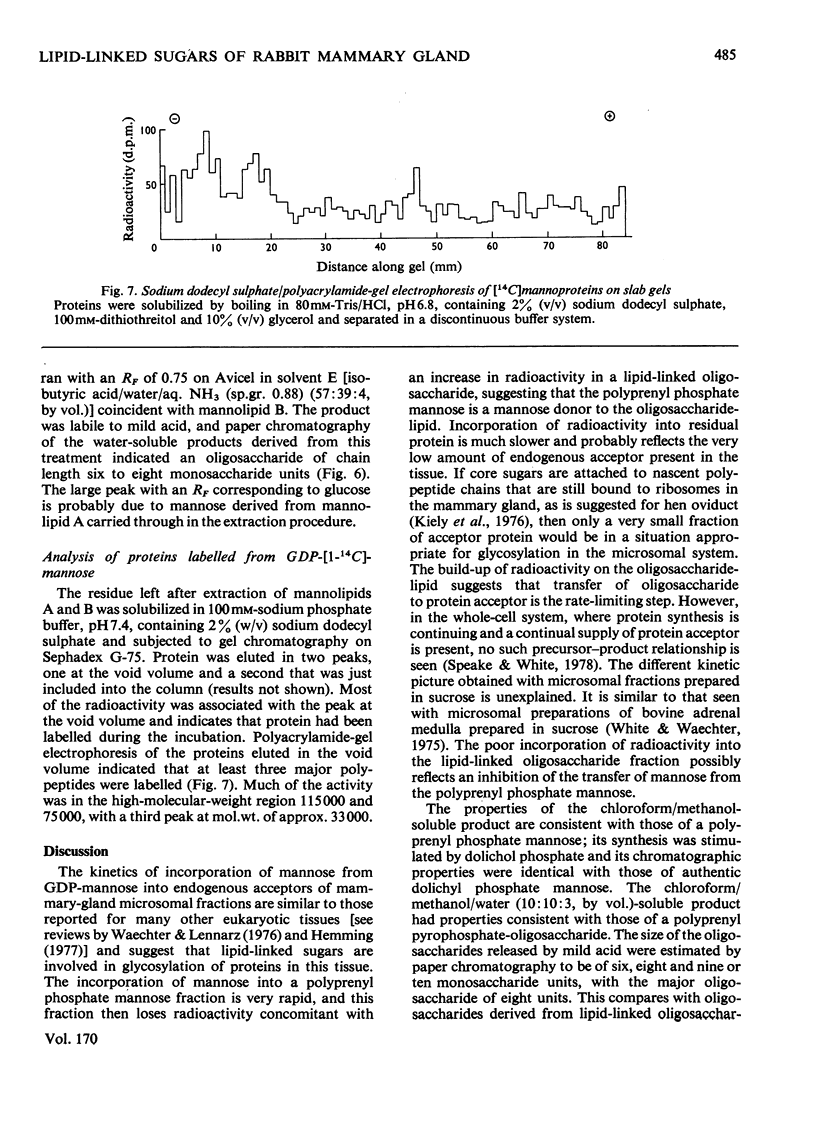
1. A lactating rabbit mammary-gland microsomal system catalysed the incorporation of mannose from GDP-[U-14C]mannose into three endogenous acceptors, (i) polyprenyl phosphate mannose, (ii) lipid-linked oligosaccharide and (iii) protein. 2. Synthesis of polyprenyl phosphate mannose was stimulated by addition of dolichol phosphate to the incubation medium and was reversed by addition of GDP. The product had properties identical with those of authentic dolichol phosphate mannose. 3. The oligosaccharides derived from acid hydrolysis of the lipid-linked oligosaccharide fraction were of six, eight and nine to ten monosaccharide units, the octasaccharide being the major species formed. The oligosaccharide appeared to be attached to the lipid via a pyrophosphate bridge, since strong alkaline hydrolysis liberated an oligosaccharide phosphate. 4. Polyprenyl phosphate mannose served as a mannose donor to lipid-linked oligosaccharides and protein. When added as exogenous substrate it gave rise to a lipid-linked oligosaccharide of about six units. 5. Incorporation of radioactivity in protein was low, but polyacrylamide-gel electrophoresis of the protein fractions indicated that polypeptides of mol.wts. 115000, 75000 and 33000 were labelled.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behrens N. H., Leloir L. F. Dolichol monophosphate glucose: an intermediate in glucose transfer in liver. Proc Natl Acad Sci U S A. 1970 May;66(1):153–159. doi: 10.1073/pnas.66.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens N. H., Parodi A. J., Leloir L. F. Glucose transfer from dolichol monophosphate glucose: the product formed with endogenous microsomal acceptor. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2857–2860. doi: 10.1073/pnas.68.11.2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts S. A., Mayer R. J. Purification and properties of 6-phosphogluconate dehydrogenase from rabbit mammary gland. Biochem J. 1975 Nov;151(2):263–270. doi: 10.1042/bj1510263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. C., Fish W. W., Hudson B. G., Ebner K. E. Isolation and characterization of rat alpha-lactalbumin: a glycoprotein. Biochim Biophys Acta. 1977 Mar 28;491(1):82–92. doi: 10.1016/0005-2795(77)90043-5. [DOI] [PubMed] [Google Scholar]
- Hemming F. W. Dolichol phosphate, a coenzyme in the glycosylation of animal membrane-bound glycoproteins. Biochem Soc Trans. 1977;5(4):1223–1231. doi: 10.1042/bst0051223. [DOI] [PubMed] [Google Scholar]
- Herscovics A., Golovtchenko A. M., Warren C. D., Bugge B., Jeanloz R. W. Mannosyltransferase activity in calf pancreas microsomes. Formation of 14C-labeled lipid-linked oligosaccharides from GDP-D-[14C]mannose and pancreatic dolichyl beta-D-[14C]mannopyranosyl phosphate. J Biol Chem. 1977 Jan 10;252(1):224–234. [PubMed] [Google Scholar]
- Kiely M. L., McKnight G. S., Schimke R. T. Studies on the attachment of carbohydrate to ovalbumin nascent chains in hen oviduct. J Biol Chem. 1976 Sep 25;251(18):5490–5495. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Gurdon J. B., Crawford L. V. Translation of encephalomyocarditis viral RNA in oocytes of Xenopus laevis. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3665–3669. doi: 10.1073/pnas.69.12.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas J. J., Waechter J., Lennarz W. J. The participation of lipid-linked oligosaccharide in synthesis of membrane glycoproteins. J Biol Chem. 1975 Mar 25;250(6):1992–2002. [PubMed] [Google Scholar]
- Lucas J. J., Waechter J., Lennarz W. J. The participation of lipid-linked oligosaccharide in synthesis of membrane glycoproteins. J Biol Chem. 1975 Mar 25;250(6):1992–2002. [PubMed] [Google Scholar]
- Parodi A. J., Staneloni R., Cantarella A. I., Leloir L. F., Behrens N. H., Carminatti H., Levy J. A. Further studies on a glycolipid formed from dolichyl-D-glucosyl monophosphate. Carbohydr Res. 1973 Feb;26(2):393–400. doi: 10.1016/s0008-6215(00)84527-9. [DOI] [PubMed] [Google Scholar]
- Robbins P. W., Krag S. S., Liu T. Effects of UDP-glucose addition on the synthesis of mannosyl lipid-linked oligosaccharides by cell-free fibroblast preparations. J Biol Chem. 1977 Mar 10;252(5):1780–1785. [PubMed] [Google Scholar]
- Speake B. K., White D. A. The formation of lipid-linked sugars as intermediates in glycoprotein synthesis in rabbit mammary gland. Biochem J. 1978 Feb 15;170(2):273–283. doi: 10.1042/bj1700273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waechter C. J., Lennarz W. J. The role of polyprenol-linked sugars in glycoprotein synthesis. Annu Rev Biochem. 1976;45:95–112. doi: 10.1146/annurev.bi.45.070176.000523. [DOI] [PubMed] [Google Scholar]
- White D. A. The biosynthesis of mannose-containing lipids as intermediates in the glycosylation of proteins of rabbit mammary gland [proceedings]. Biochem Soc Trans. 1977;5(4):1022–1023. doi: 10.1042/bst0051022. [DOI] [PubMed] [Google Scholar]
- White D. A., Waechter C. J. A mannosyl-carrier lipid of bovine adrenal meddulla and rat parotid. Biochem J. 1975 Mar;146(3):645–651. doi: 10.1042/bj1460645. [DOI] [PMC free article] [PubMed] [Google Scholar]


