Abstract
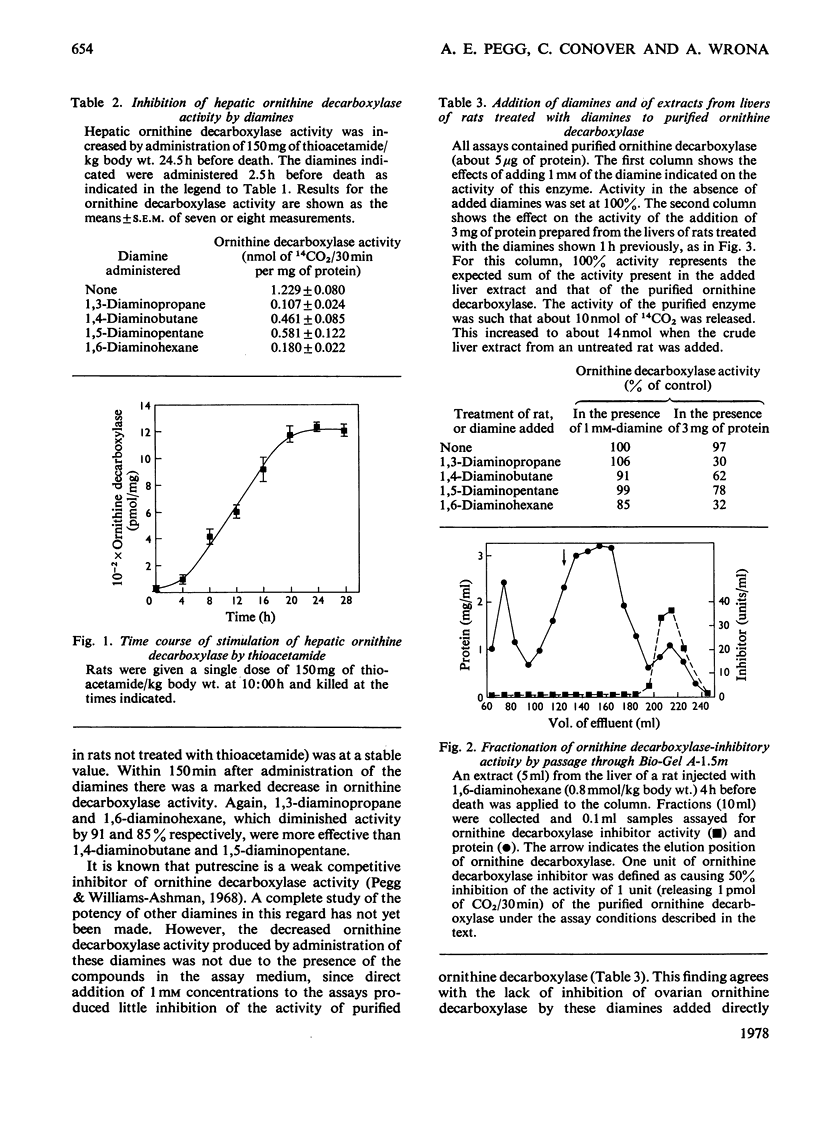
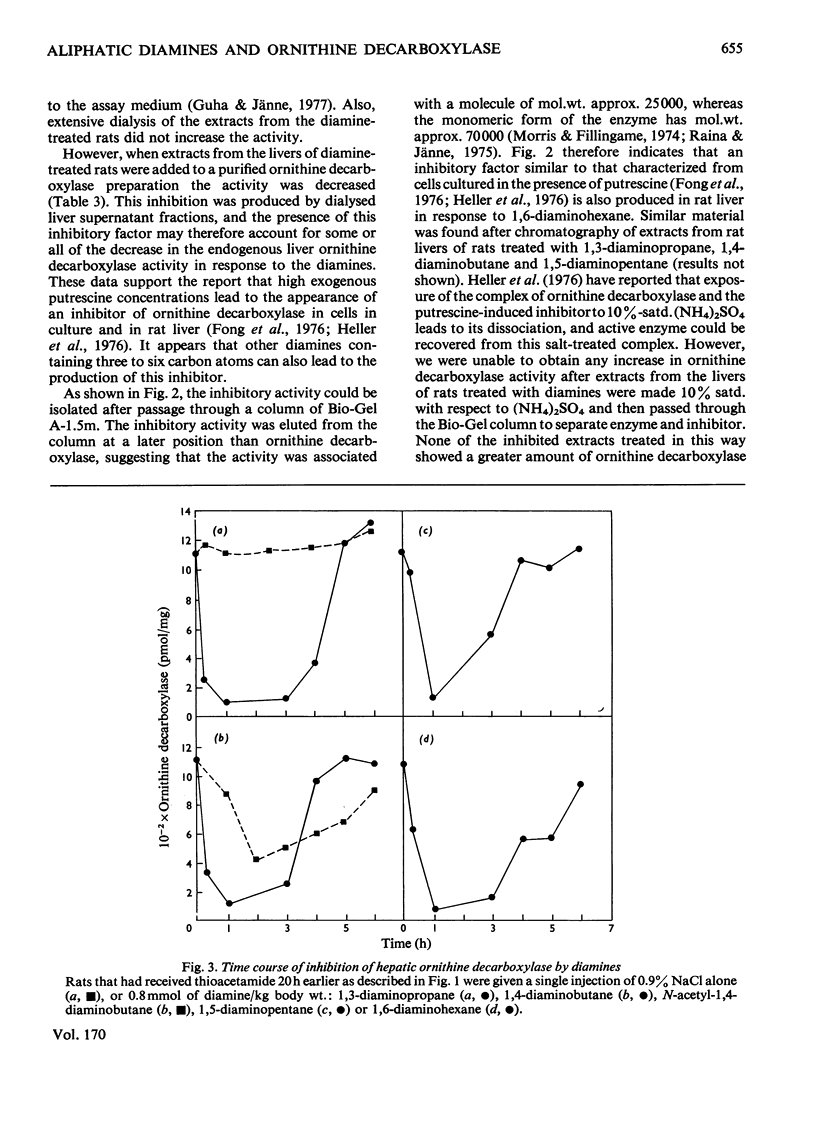
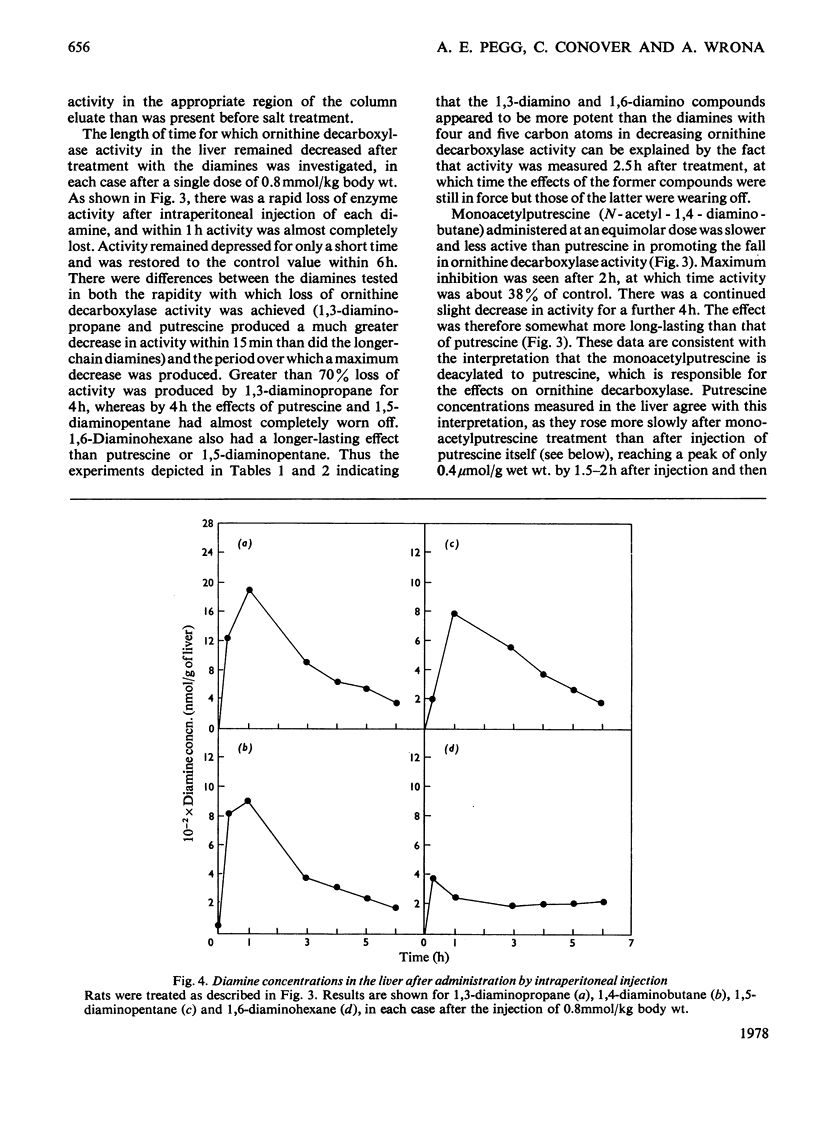
Rat liver ornithine decarboxylase activity was decreased by administration of putrescine (1,4-diaminobutane) or other diamines, including 1,3-diaminopropane, 1,5-diaminopentane and 1,6-diaminohexane. This effect was seen in control rats and in rats in which hepatic ornithine decarboxylase activity had been increased by administration of growth hormone (somatotropin) or thioacetamide. Loss of activity was not dependent on the conversion of putrescine into polyamines and was short-lived. Within 6h after intraperitoneal administration of 0.8 mmol/kg body wt., ornithine decarboxylase activity had returned to normal values. This return correlated with the rapid loss of the diamines from the liver, and the decrease in activity could be slightly prolonged by treatment with aminoguanidine, a diamine oxidase inhibitor. A decrease in ornithine decarboxylase activity by these diamines was accompanied by the accumulation in the liver of a nondiffusible inhibitor that decreased the activity of a purified ornithine decarboxylase preparation. The possibility that administration of non-physiological diamines that are not converted into polyamines might be useful for the inhibition of polyamine synthesis is discussed.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradsley W. G., Crabbe M. J., Scott I. V. The amine oxidases of human placenta and pregnancy plasma. Biochem J. 1974 Apr;139(1):169–181. doi: 10.1042/bj1390169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J. L., Fuller J. L. Protein inhibitor of ornithine decarboxylase does not account for effect of putrescine on 3T3 cells. Biochem Biophys Res Commun. 1976 Dec 6;73(3):785–790. doi: 10.1016/0006-291x(76)90878-0. [DOI] [PubMed] [Google Scholar]
- Clark J. L., Fuller J. L. Regulation of ornithine decarboxylase in 3T3 cells by putrescine and spermidine: indirect evidence for translational control. Biochemistry. 1975 Oct 7;14(20):4403–4409. doi: 10.1021/bi00691a010. [DOI] [PubMed] [Google Scholar]
- Fausto N. RNA and amine synthesis in the liver of rats given injections of thioacetamide. Cancer Res. 1970 Jul;30(7):1947–1952. [PubMed] [Google Scholar]
- Fong W. F., Heller J. S., Canellakis E. S. The appearance of an ornithine decarboxylase inhibitory protein upon the addition of putrescine to cell cultures. Biochim Biophys Acta. 1976 Apr 23;428(2):456–465. doi: 10.1016/0304-4165(76)90054-4. [DOI] [PubMed] [Google Scholar]
- Guha S. K., Jänne J. Inhibition of ornithine decarboxylase in vivo in rat ovary. Biochem Biophys Res Commun. 1977 Mar 7;75(1):136–142. doi: 10.1016/0006-291x(77)91300-6. [DOI] [PubMed] [Google Scholar]
- Heller J. S., Fong W. F., Canellakis E. S. Induction of a protein inhibitor to ornithine decarboxylase by the end products of its reaction. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1858–1862. doi: 10.1073/pnas.73.6.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölttä E., Hannonen P., Pispa J., Jänne J. Effect of methylglyoxal bis(guanylhydrazone) on polyamine metabolism in normal and regenerating rat liver and rat thymus. Biochem J. 1973 Nov;136(3):669–676. doi: 10.1042/bj1360669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölttä E. Immunochemical demonstration of increased accumulation of ornithine carboxylase in rat liver after partial hepatectomy and growth hormone induction. Biochim Biophys Acta. 1975 Aug 13;399(2):420–427. doi: 10.1016/0304-4165(75)90270-6. [DOI] [PubMed] [Google Scholar]
- Hölttä E. Oxidation of spermidine and spermine in rat liver: purification and properties of polyamine oxidase. Biochemistry. 1977 Jan 11;16(1):91–100. doi: 10.1021/bi00620a015. [DOI] [PubMed] [Google Scholar]
- Inoue H., Kato Y., Takigawa M., Adachi K., Takeda Y. Effect of DL-alpha-hydrazino-delta-aminovaleric acid, an inhibitor of ornithine decarboxylase, on polyamine metabolism in isoproterenol-stimulated mouse parotid glands. J Biochem. 1975 Apr;77(4):879–893. doi: 10.1093/oxfordjournals.jbchem.a130796. [DOI] [PubMed] [Google Scholar]
- Jefferson L. S., Pegg A. E. Studies on ornithine decarboxylase activity in the isolated perfused rat liver. Biochim Biophys Acta. 1977 Sep 15;484(1):177–187. doi: 10.1016/0005-2744(77)90123-1. [DOI] [PubMed] [Google Scholar]
- Jänne J., Hölttä E. Regulation of ornithine decarboxylase activity by putrescine and spermidine in rat liver. Biochem Biophys Res Commun. 1974 Nov 27;61(2):449–456. doi: 10.1016/0006-291x(74)90977-2. [DOI] [PubMed] [Google Scholar]
- Jänne J. Studies on the biosynthetic pathway of polyamines in rat liver. Acta Physiol Scand Suppl. 1967;300:1–71. [PubMed] [Google Scholar]
- Kallio A., Löfman M., Pösö H., Jänne J. Inhibition of ornithine decarboxylase by diamines in regenerating rat liver. Evidence for direct action on the accumulation of the enzyme protein. FEBS Lett. 1977 Jul 1;79(1):195–199. [PubMed] [Google Scholar]
- Kay J. E., Lindsay V. J. Control of ornithine decarboxylase activity in stimulated human lymphocytes by putrescine and spermidine. Biochem J. 1973 Apr;132(4):791–796. doi: 10.1042/bj1320791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mamont P. S., Böhlen P., McCann P. P., Bey P., Schuber F., Tardif C. Alpha-methyl ornithine, a potent competitive inhibitor of ornithine decarboxylase, blocks proliferation of rat hepatoma cells in culture. Proc Natl Acad Sci U S A. 1976 May;73(5):1626–1630. doi: 10.1073/pnas.73.5.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann P. P., Tardif C., Mamont P. S. Regulation of ornithine decarboxylase by ODC-antizyme in HTC cells. Biochem Biophys Res Commun. 1977 Apr 25;75(4):948–954. doi: 10.1016/0006-291x(77)91474-7. [DOI] [PubMed] [Google Scholar]
- Morris D. R., Fillingame R. H. Regulation of amino acid decarboxylation. Annu Rev Biochem. 1974;43(0):303–325. doi: 10.1146/annurev.bi.43.070174.001511. [DOI] [PubMed] [Google Scholar]
- Newton N. E., Abdel-Monem M. M. Inhibitors of polyamine biosynthesis. 4. Effects of alpha-methyl-(+/-)-ornithine and methylglyoxal bis(guanylhydrazone) on growth and polyamine content of L1210 leukemic cells of mice. J Med Chem. 1977 Feb;20(2):249–253. doi: 10.1021/jm00212a012. [DOI] [PubMed] [Google Scholar]
- Ono M., Inoue H., Takeda Y. Effect of thioamide derivatives on induction of ornithine decarboxylase in rat liver. Biochim Biophys Acta. 1973 Apr 28;304(2):495–504. doi: 10.1016/0304-4165(73)90269-9. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Conover C. Irreversible inhibition of mamalian and yeast S-adenosylmethionine decarboxylase by 1,1'- (methylethanediylidenedinitrilo)-bis (3-aminoguanidine). Biochem Biophys Res Commun. 1976 Apr 5;69(3):766–774. doi: 10.1016/0006-291x(76)90941-4. [DOI] [PubMed] [Google Scholar]
- Pegg A. E. Inhibition of spermidine formation in rat liver and kidney by methylglyoxal bis(guanylhydrazone). Biochem J. 1973 Mar;132(3):537–540. doi: 10.1042/bj1320537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., Lockwood D. H., Williams-Ashman H. G. Concentrations of putrescine and polyamines and their enzymic synthesis during androgen-induced prostatic growth. Biochem J. 1970 Mar;117(1):17–31. doi: 10.1042/bj1170017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., Williams-Ashman H. G. Biosynthesis of putrescine in the prostate gland of the rat. Biochem J. 1968 Jul;108(4):533–539. doi: 10.1042/bj1080533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., Williams-Ashman H. G. On the role of S-adenosyl-L-methionine in the biosynthesis of spermidine by rat prostate. J Biol Chem. 1969 Feb 25;244(4):682–693. [PubMed] [Google Scholar]
- Pöso H., Jänne J. Inhibition of ornithine decarboxylase activity and spermidine accumulation in regenerating rat liver. Biochem Biophys Res Commun. 1976 Apr 19;69(4):885–892. doi: 10.1016/0006-291x(76)90456-3. [DOI] [PubMed] [Google Scholar]
- Pösö H., Jänne J. Inhibition of polyamine accumulation and deoxyribonucleic acid synthesis in regenerating rat liver. Biochem J. 1976 Aug 15;158(2):485–488. doi: 10.1042/bj1580485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina A., Jänne J. Physiology of the natural polyamines putrescine, spermidine and spermine. Med Biol. 1975 Jun;53(3):121–147. [PubMed] [Google Scholar]
- Relyea N., Rando R. R. Potent inhibition of ornithine decarboxylase by beta,gamma unsaturated substrate analogs. Biochem Biophys Res Commun. 1975 Nov 3;67(1):392–398. doi: 10.1016/0006-291x(75)90328-9. [DOI] [PubMed] [Google Scholar]
- Seiler N., Eichentopf B. 4-aminobutyrate in mammalian putrescine catabolism. Biochem J. 1975 Nov;152(2):201–210. doi: 10.1042/bj1520201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler N., al-Therib M. J. Acetyl-CoA: 1,4-diaminobutane N-acetyltransferase. Occurrence in vertebrate organs and subcellular localization. Biochim Biophys Acta. 1974 Jul 4;354(2):206–212. doi: 10.1016/0304-4165(74)90007-5. [DOI] [PubMed] [Google Scholar]
- Shindler J. S., Bardsley W. G. Human kidney diamine oxidase: inhibition studies. Biochem Pharmacol. 1976 Dec 15;25(24):2689–2694. doi: 10.1016/0006-2952(76)90258-6. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. 1,4-Diaminobutane (putrescine), spermidine, and spermine. Annu Rev Biochem. 1976;45:285–306. doi: 10.1146/annurev.bi.45.070176.001441. [DOI] [PubMed] [Google Scholar]
- Tabor H., Tabor C. W. Biosynthesis and metabolism of 1,4-diaminobutane, spermidine, spermine, and related amines. Adv Enzymol Relat Areas Mol Biol. 1972;36:203–268. doi: 10.1002/9780470122815.ch7. [DOI] [PubMed] [Google Scholar]
- Williams-Ashman H. G., Corti A., Tadolini B. On the development of specific inhibitors of animal polyamine biosynthetic enzymes. Ital J Biochem. 1976 Jan-Feb;25(1):5–32. [PubMed] [Google Scholar]
- Williams-Ashman H. G., Schenone A. Methyl glyoxal bis(guanylhydrazone) as a potent inhibitor of mammalian and yeast S-adenosylmethionine decarboxylases. Biochem Biophys Res Commun. 1972 Jan 14;46(1):288–295. doi: 10.1016/0006-291x(72)90661-4. [DOI] [PubMed] [Google Scholar]


