Abstract
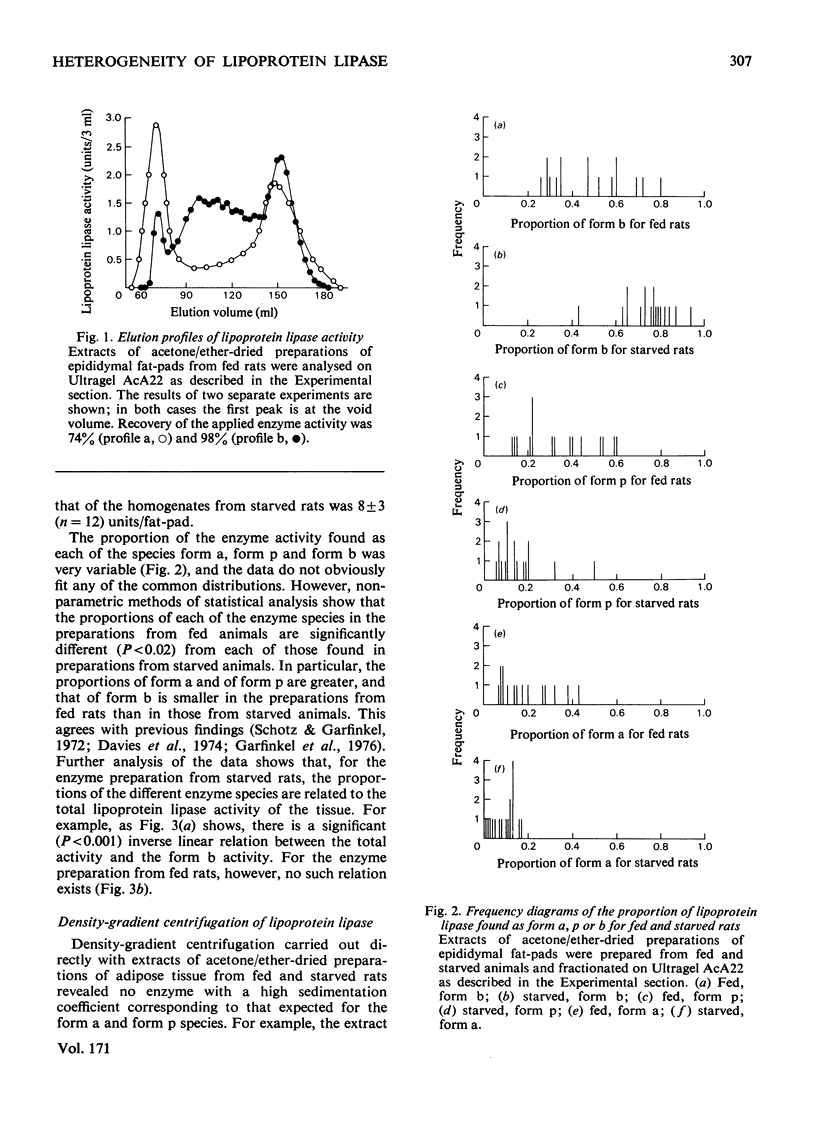
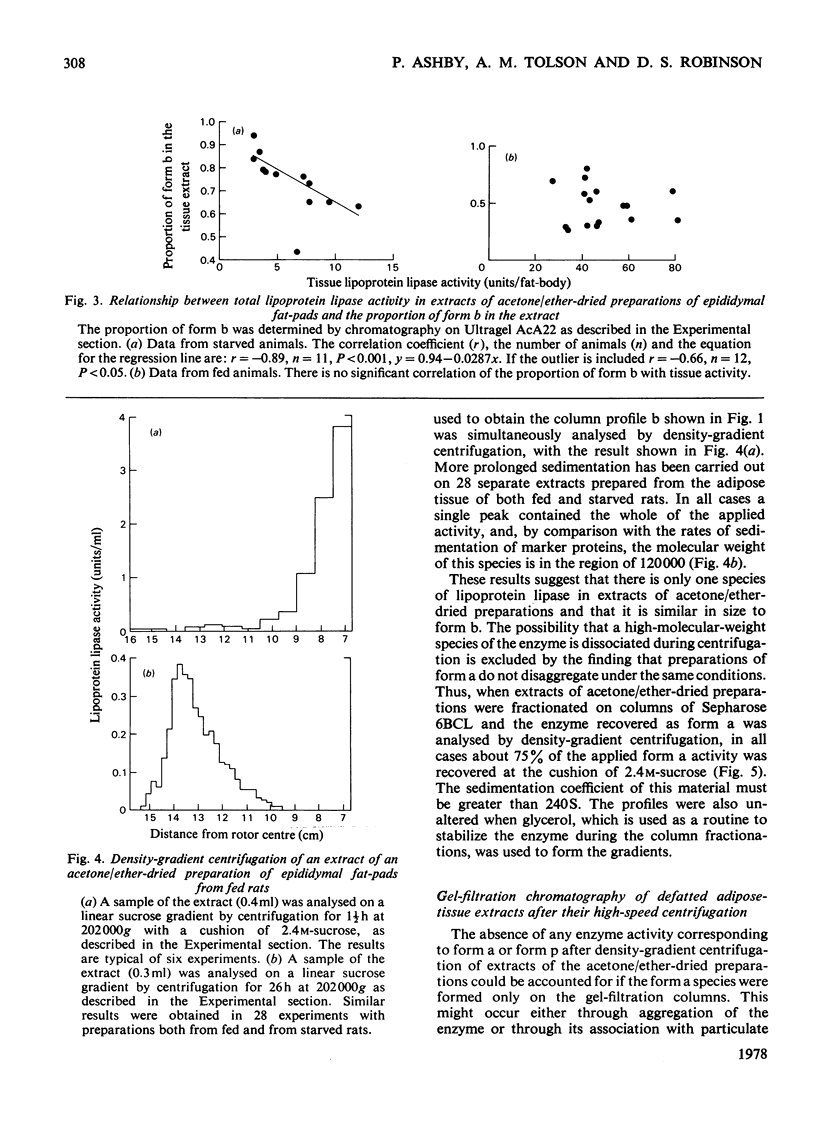
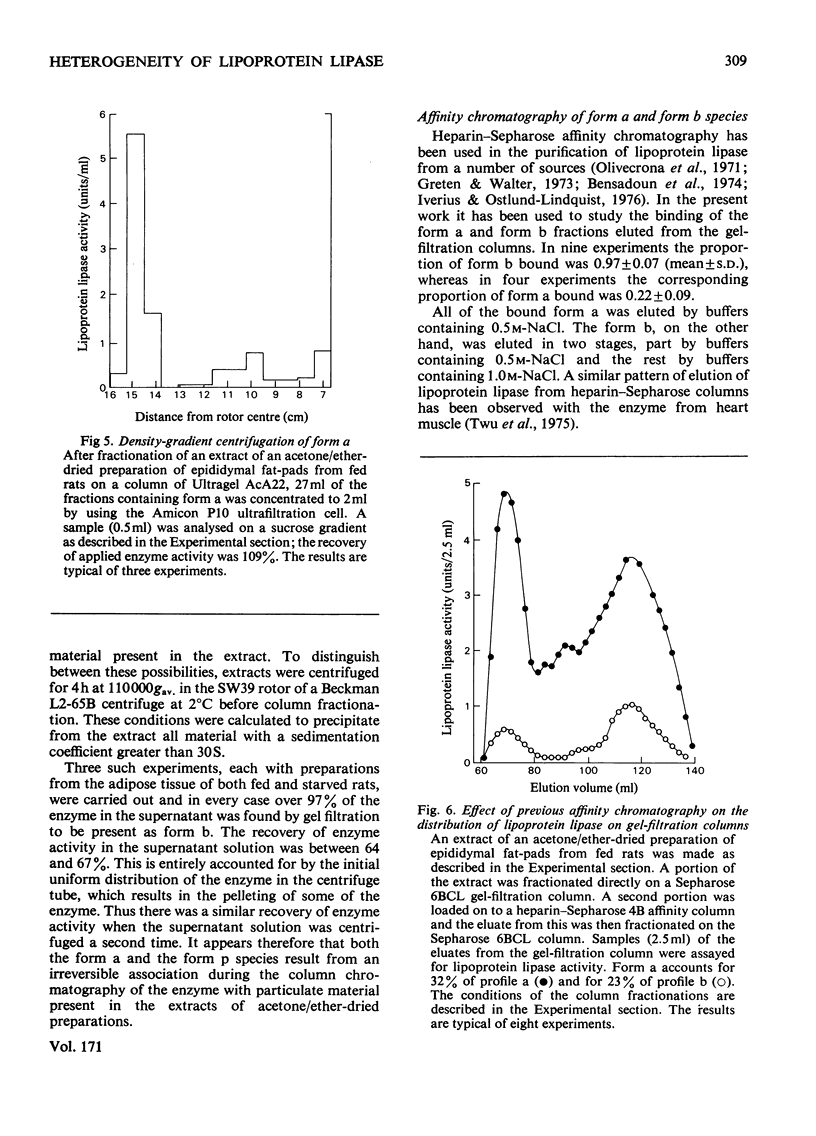
Lipoprotein lipase is heterogeneous, and it was suggested that the enzyme in adipose tissue is transformed from a species of mol. wt. approx. 120000 to forms of much higher molecular weight as it is secreted from the fat-cell. This paper demonstrates that the forms of higher molecular weight are probably artifacts. Enzyme preparations were characterized by gel filtration, by density-gradient centrifugation and by affinity chromatography. The results indicate that the enzyme forms of mol. wt. greater than 120000 result from an association of the enzyme with particulate material. It is therefore necessary to reconsider schemes that have recently been proposed for the synthesis and export of lipoprotein lipase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bensadoun A., Ehnholm C., Steinberg D., Brown W. V. Purification and characterization of lipoprotein lipase from pig adipose tissue. J Biol Chem. 1974 Apr 10;249(7):2220–2227. [PubMed] [Google Scholar]
- Cryer A., Foster B., Wing D. R., Robinson D. S. The effect of cycloheximide on adipose-tissue clearing-factor lipase. Biochem J. 1973 Apr;132(4):833–836. doi: 10.1042/bj1320833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham V. J., Robinson D. S. Clearing-factor lipase in adipose tissue. Distinction of different states of the enzyme and the possible role of the fat cell in the maintenance of tissue activity. Biochem J. 1969 Apr;112(2):203–209. doi: 10.1042/bj1120203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnall D. W., Klotz I. M. Subunit constitution of proteins: a table. Arch Biochem Biophys. 1975 Feb;166(2):651–682. doi: 10.1016/0003-9861(75)90432-4. [DOI] [PubMed] [Google Scholar]
- Davies P., Cryer A., Robinson D. S. Hormonal control of adipose tissue clearing factor lipase activity. FEBS Lett. 1974 Sep 1;45(1):271–275. doi: 10.1016/0014-5793(74)80860-4. [DOI] [PubMed] [Google Scholar]
- Davies P., Robinson D. S. Stability of clearing-factor lipase in rat adipose tissue. Biochem J. 1973 Oct;136(2):437–439. doi: 10.1042/bj1360437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel A. G., Nilsson-ehle P., Schotz M. C. Regulation of lipoprotein lipase. Induction by insulin. Biochim Biophys Acta. 1976 Feb 23;424(2):264–273. doi: 10.1016/0005-2760(76)90194-6. [DOI] [PubMed] [Google Scholar]
- Garfinkel A. S., Baker N., Schotz M. C. Relationship of lipoprotein lipase activity to triglyceride uptake in adipose tissue. J Lipid Res. 1967 May;8(3):274–280. [PubMed] [Google Scholar]
- Garfinkel A. S., Schotz M. C. Separation of molecular species of lipoprotein lipase from adipose tissue. J Lipid Res. 1972 Jan;13(1):63–68. [PubMed] [Google Scholar]
- Greten H., Walter B. Purification of rat adipose tissue lipoprotein lipase. FEBS Lett. 1973 Sep 1;35(1):36–40. doi: 10.1016/0014-5793(73)80571-x. [DOI] [PubMed] [Google Scholar]
- Herscovics A. Biosynthesis of thyroglobulin: incorporation of [3H] fucose into proteins by rat thyroids in vitro. Biochem J. 1970 Apr;117(2):411–413. doi: 10.1042/bj1170411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverius P. H. Coupling of glycosaminoglycans to agarose beads (sepharose 4B). Biochem J. 1971 Oct;124(4):677–683. doi: 10.1042/bj1240677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverius P. H., Ostlund-Lindqvist A. M. Lipoprotein lipase from bovine milk. Isolation procedure, chemical characterization, and molecular weight analysis. J Biol Chem. 1976 Dec 25;251(24):7791–7795. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Nilsson-Ehle P., Garfinkel A. S., Schotz M. C. Intra- and extracellular forms of lipoprotein lipase in adipose tissue. Biochim Biophys Acta. 1976 Apr 22;431(1):147–156. doi: 10.1016/0005-2760(76)90269-1. [DOI] [PubMed] [Google Scholar]
- Olivecrona T., Egelrud T., Iverius P. H., Lindahl U. Evidence for an ionic binding of lipoprotein lipase to heparin. Biochem Biophys Res Commun. 1971 May 7;43(3):524–529. doi: 10.1016/0006-291x(71)90645-0. [DOI] [PubMed] [Google Scholar]
- ROBINSON D. S. THE CLEARING FACTOR LIPASE AND ITS ACTION IN THE TRANSPORT OF FATTY ACIDS BETWEEN THE BLOOD AND TISSUES. Adv Lipid Res. 1963;1:133–182. doi: 10.1016/b978-1-4831-9937-5.50010-7. [DOI] [PubMed] [Google Scholar]
- Robinson D. S., Cryer A., Davies P. The role of clearing-factor lipase (lipoprotein lipase) in the transport of plasma triglycerides. Proc Nutr Soc. 1975 Dec;34(3):211–215. doi: 10.1079/pns19750041. [DOI] [PubMed] [Google Scholar]
- Salaman M. R., Robinson D. S. Clearing-factor lipase in adipose tissue. A medium in which the enzyme activity of tissue from starved rats increases in vitro. Biochem J. 1966 Jun;99(3):640–647. doi: 10.1042/bj0990640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schotz M. C., Garfinkel A. S. Effect of nutrition on species of lipoprotein lipase. Biochim Biophys Acta. 1972 Aug 11;270(4):472–478. doi: 10.1016/0005-2760(72)90112-9. [DOI] [PubMed] [Google Scholar]
- Schotz M. C., Garfinkel A. S. The effect of puromycin and actinomycin on carbohydrate-induced lipase activity in rat adipose tissue. Biochim Biophys Acta. 1965 Jul 7;106(1):202–205. doi: 10.1016/0005-2760(65)90109-8. [DOI] [PubMed] [Google Scholar]
- Stewart J. E., Schotz M. C. Studies on release of lipoprotein lipase activity from fat cells. J Biol Chem. 1971 Sep 25;246(18):5749–5753. [PubMed] [Google Scholar]
- Twu J. S., Garfinkel A. S., Schotz M. C. Rat heart lipoprotein lipase. Atherosclerosis. 1975 Nov-Dec;22(3):463–472. doi: 10.1016/0021-9150(75)90025-8. [DOI] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]
- Wilson J. E. Studies on the molecular weight and lipoprotein nature of glucose-6-phosphate solubilized rat brain hexokinase. Arch Biochem Biophys. 1973 Jan;154(1):332–340. doi: 10.1016/0003-9861(73)90065-9. [DOI] [PubMed] [Google Scholar]
- Wing D. R., Robinson D. S. Clearing-factor lipase in adipose tissue. A possible role of adenosine 3',5'-(cyclic)-monophosphate in the regulation of its activity. Biochem J. 1968 Oct;109(5):841–849. doi: 10.1042/bj1090841. [DOI] [PMC free article] [PubMed] [Google Scholar]


