Abstract
The genus Polyscias, part of the Araliaceae family, is known for its significant ornamental and medicinal value, as well as its rich variety of metabolites. These plants are primarily found in tropical regions, particularly in Southeast Asia and the Pacific islands. The diverse geographical environments have led to the emergence of many unique and endangered species, although there is limited genomic information available about them. In this study, we generated high‐quality reference genomes for three endangered species: two that are endemic to Hawaiʻi, Polyscias cf. bisattenuata and Polyscias lallanii, and one more widespread species, Polyscias macgillivrayi. We identified a total of 51,083, 60,881, and 29,060 genes in these three species, respectively. Whole‐genome duplication analysis indicated that all three species underwent a common duplication event. By examining the phylogenetic and structural characteristics of the terpene synthase gene family in these species and closely related species, we identified several gene clusters that play crucial roles in metabolite synthesis. A variety of mono‐ and sesquiterpenoids were detected, with several of these compounds having been validated in previous studies. Our findings provide a foundation for further genetic and biochemical investigations of Polyscias, which may aid in the conservation of these endangered species.
Core Ideas
Three high‐quality reference genomes of Polyscias species were assembled for phylogenetic analysis.
Terpene synthase‐related genes and metabolites of the three species and closely related species were identified and analyzed.
Phylogenetic analyses clarified relationships with other plant species.
Abbreviations
- BGCs
biosynthesis gene clusters
- BUSCO
Benchmarking Universal Single‐Copy Orthologs
- CCS
circular consensus sequencing
- GO
Gene Ontology
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- TE
transposable element
- TPS
terpene synthase
- WGD
whole‐genome duplication
1. INTRODUCTION
The genus Polyscias J. R. Forst & G. Forst (Araliaceae Juss.) contains 183 species (Ashmawy et al., 2020) according to the latest descriptions based on molecular data (Lowry & Plunkett, 2010). Polyscias species are found mainly in Southeast Asia and the Pacific region as well as in tropical Africa. Some species of Polyscias, such as Polyscias scutellaria, have significant medicinal value (Quattrocchi, 2012). Biological studies have demonstrated that extracts and bioactive compounds from Polyscias possess various activities, including antibacterial, antifungal, cytotoxic, immunostimulatory, wound‐healing, and anti‐asthmatic activities (Huan et al., 1998; Sugimoto et al., 2017). Most of the described activities have been attributed to terpenoids, particularly triterpenoids (Eaton et al., 2015; Mitaine‐Offer et al., 2004), of which saponins have been shown to be the major components (Ashmawy et al., 2020). Several species in the genus, such as Polyscias guilfoylei and Polyscias balfouriana, produce essential oils (Ashmawy et al., 2020; Kurup et al., 2020); these oils consist mainly of phenylpropanoids and mono‐ and sesquiterpenoids involved in chemical communication and defenses (Ashmawy et al., 2020; Ninkuu et al., 2021). In an ecological context, these compounds contribute to the interactions among plants, herbivores, and microorganisms, influencing ecosystem dynamics, and thus often play a crucial role in species survival (Holopainen et al., 2013; Martinez‐Swatson et al., 2020).
Smaller terpenoids, such as mono‐ and sesquiterpenoids, are derived from the activity of terpene synthases (TPSs) (Karunanithi & Zerbe, 2019). The TPS gene family can be divided into seven subfamilies according to phylogenetic analysis, namely, TPS‐a, TPS‐b, TPS‐c, TPS‐d, TPS‐e/f, TPS‐g, and TPS‐h (Yan et al., 2023). They have been identified at the genomic level in various plant species (Jia et al., 2022). The TPS‐a subfamily in general encodes only sesqui‐TPSs (Martin et al., 2010), whereas the TPS‐b and TPS‐g subfamilies in general encode mainly enzymes for monoterpenoid biosynthesis (Williams et al., 1998); these three subfamilies are found only in angiosperms. The TPS‐c subfamily is found in all plants (Chen et al., 2011), whereas TPS‐ds are gymnosperm specific, and the TPS‐e/f subfamily is found mainly in vascular plants (Chen et al., 2011). Collectively, these TPS genes produce the vast diversity of terpenoids found in plants (Jia et al., 2022). Aside from these smaller terpenoids, triterpenoids are synthesized via the conjugation of two farnesyl diphosphates into squalene followed by various cyclization reactions. Plant triterpenoids constitute one of the largest subclasses of terpenoids, with more than 14,000 known structures and over 100 distinct cyclical triterpene scaffolds, and play crucial roles in plant defense and development (Cárdenas et al., 2019).
In addition to research on pharmacological activities, studies on Araliaceae plants have focused on species evolution and diversification mechanisms (Kang et al., 2023). Through karyotype analysis, researchers have shown that Araliaceae plants may have undergone extensive polyploidization events, which could have driven rapid diversification within the family (Li et al., 2021). However, the understanding of karyotype evolution and evolutionary mechanisms in Araliaceae plants remains limited due to a lack of genomic data (Yi et al., 2004). No reference genome has been published for Polyscias, and only a few genomes have been published, even for the commercially important ginseng species in the Araliaceae family.
A few Polyscias species, such as P. fruticosa (L.) Harms, have been introduced in many countries and are commonly cultivated in tropical and subtropical areas (and grown as indoor plants in other parts of the world) for ornamental purposes (Do et al., 2021). However, many Polyscias species are endemic to one or a few Pacific Islands, and many are endangered according to the IUCN Red List of Threatened Species (IUCN, 2024). For example, Polyscias cf. bisattenuata (Scherff) Lowry & G. M. Plunkett is an endangered tree species endemic to the Hawaiian island of Kauaʻi. It is occasionally found growing on slopes and steep ridges between 400 and 700 meters. Known locally as ʻohe mauka, it is an extremely rare species with only three known populations and fewer than 30 individuals remaining in the wild, which has led to focused restoration efforts by the National Tropical Botanical Garden (NTBG) and the Hawaiʻi Plant Extinction Prevention Program (PEPP) (Tangalin, 2015) and a large conservation project initiated to save the species. To prevent extinction, which is the first milestone in species recovery, more than 3700 individuals have been reintroduced; although the flowering individuals have been outplanted, no natural recruitment has been observed or reported, and the success and future stability of these reintroductions is unclear (Supple & Shapiro, 2018; US Fish and Wildlife Service, 2020). Polyscias cf. bisattenuata is a long‐lived perennial tree species that is an obligate outcrosser.
Polyscias macgillivrayi (Benth.) Harms is a shrub or small tree that grows to 3–4 m in height, with plants up to 15 m in height observed. It usually has a single bole with only a few branches. Its native range spans from New Guinea to the western Pacific, and it is found primarily in the wet tropical biotopes (Plants of the World Online, n.d.). The leaves of MacGillivray's aralia are also harvested from the wild for local use, as the leaves have a strong curry‐like odor when dried (French, 1986).
Polyscias lallanii R. Kr. Singh & Sanjeet Kumar (Singh, 2024), commonly known as false ‘ohe, is a small tree that grows to a height of 7–8 m, featuring a straight trunk, spreading branches, and smooth gray bark. Polyscias lallanii is endemic to Kauaʻi and is also an extremely rare and endangered species that thrives in moist forests at elevations ranging from 130 to 430 m (Wagner et al., 2023).
In the present study, we obtained three genome assemblies from a combination of MGISEQ short‐read and PacBio HiFi data. This study provides the first genomic insight into the genus Polyscias. After annotating the assembled genome, we constructed a phylogenetic tree to assess the evolutionary history of the genus Polyscias with respect to key species in the families Araliaceae and Apiaceae. Further, we analyzed the TPS genes and also identified a several of mono‐ and sesquiterpenoids, where must was previously identified. The assembled genomic data establish a basis for understanding the differentiation of these species and will facilitate future genetic studies and protect these endangered species.
2. MATERIALS AND METHODS
2.1. Plant material
The plant materials of eight Polyscias (Araliaceae) species was collected from Kauaʻi, Hawaiʻi, United States. To limit the impacts of sampling from wild populations, plant materials of known provenance were sourced when possible from the living collection of the NTBG or from wild populations when living collections were not available and a small branchlet could safely be sampled from the tree. When possible, samples of different tissues (twigs, leaves, flowers) were collected for mRNA analysis. Identification was performed by the authors, and in a few situations, a new voucher was deposited at Herbarium PTBG of the NTBG on Kauaʻi. For most collections, the voucher was already established in the collection of NTBG. Only general locality information is provided for wild collections following local regulations to protect rare and endangered species. Initially, the aim of the study was to perform genome sequencing of all eight species, but owing to various challenges, only three genome sequences were obtained; however, the mRNA data of all eight species were included to support the discussion of genomic evolution and terpenoid biochemistry. The PLANTS of HAWAIʻI (https://plantsofhawaii.org/), Kew Garden (https://naturalhistory2.si.edu/botany/), and National Museum of Natural History (NMNH) (https://collections.nmnh.si.edu/) databases contain photographs, specimens, and information on the distribution of the Polyscias species for further studies:
Po. cf. bisattenuata (Sherff) Lowry & G. M. Plunkett, collected on November 24, 2020, NTBG living collection accession 070821‐004, voucher: Lorence 10488 (PTBG). IUCN Red List status: Critically Endangered (Tangalin, 2015).
Polyscias kavaiensis (H. Mann) Lowry & G. M. Plunkett, collected on May 11, 2022 in Kokee, Waimea, Kauaʻi, voucher: K. R. Wood et al. 18970 (PTBG). Estimated IUCN Red List status: Vulnerable (Rønsted et al., 2022).
Po. macgillivrayi (Benth.) Harms, collected on January 29, 2021 and May 19, 2022, NTBG living collection accession 970415‐005, voucher: D. Lorence 10840 (PTBG). IUCN Red List status: Least Concern (Jimbo, 2022).
Polyscias oahuensis (A. Gray) Lowry & G.M. Plunkett, collected on May 20, 2022 in Upper Limahuli Preserve, voucher: U. Nagendra (PTBG). Estimated IUCN Red List Status: Vulnerable (Rønsted et al., 2022).
Po. lallanii R. Kr. Singh & Sanjeet (syn. Polyscias racemosa [C.N. Forbes] Lowry & G. M. Plunkett), collected on November 24, 2020, NTBG living collection accession 910244‐001, voucher: D. Lorence & N. Rønsted 10831 (PTBG). IUCN Red List status: Critically Endangered (Adams, 2016b).
Polyscias sandwicensis (A. Gray) Lowry & G. M. Plunkett, collected on November 24, 2020 and October 5, 2022, NTBG living collection accession 920160‐002, voucher: D. Lorence & N. Rønsted 10832 (PTBG). Estimated IUCN Red List status: Vulnerable (Rønsted et al., 2022).
Polyscias waialealae (Rock) Lowry & G.M. Plunkett, collected on May 11, 2022, in Kokee, Hanalei, Kauaʻi, voucher: K. R. Wood et al. 18969 (PTBG). IUCN Red List Status: Endangered (Adams, 2016c).
Polyscias waimeae (Wawra) Lowry & G. M. Plunkett, collected on May 11, 2022, in Kokee, Hanalei, Kauaʻi, voucher: T. Flynn 9322 (PTBG). IUCN Red List status: Endangered (Adams, 2016a).
Core Ideas
Three high‐quality reference genomes of Polyscias species were assembled for phylogenetic analysis.
Terpene synthase‐related genes and metabolites of the three species and closely related species were identified and analyzed.
Phylogenetic analyses clarified relationships with other plant species.
2.2. DNA extraction and sequencing
High‐quality genomic DNA was extracted from fresh leaf tissues (Table S1) via the DNeasy Plant Mini Kit (Qiagen). The quality of the extracted genomic DNA was evaluated via gel electrophoresis and a NanoDrop‐2000 spectrophotometer (Thermo Scientific). Approximately 5 µg of DNA was used to construct short DNA insert (∼350 bp) libraries via the MGIEasy Universal DNA Library Prep Kit. The libraries were sequenced on the DNBSEQ‐T7 platform in paired‐end (PE) mode with a read length of 150 bp (Table S2). We prepared a 20‐kb single‐molecule real‐time (SMRT) library using the SMRTbell Prep Kit 2.0, and sequencing was performed on the PacBio Sequel II platform at the Beijing Genomics Institute‐Shenzhen (BGI‐Shenzhen) (Table S3).
2.3. RNA extraction and sequencing
Samples of leaf, stem, and flower tissues (Table S1) were utilized for RNA extraction via an RNeasy PowerWater Kit (Qiagen). The MGIEasy RNA Directional Library Prep Kit (MGI) was utilized to construct RNA libraries, with approximately 1–2 µg of total RNA from each tissue sample used. Polyscias cf. bisattenuata and Po. lallanii libraries were subsequently subjected to sequencing via DNBSEQ‐G400, generating 150‐bp PE reads; Po. macgillivrayi libraries were subsequently subjected to sequencing via DNBSEQ‐G400, generating 100‐bp PE reads (Tables S4 and S5).
2.4. Genome assembly
High‐accuracy circular consensus sequencing (CCS) data were assembled via HiFiasm (v0.14) (Cheng et al., 2022) to obtain the genome sequence. Purge_Dups (v1.2.5) (Guan et al., 2020) was used to remove duplicates caused by heterozygosity in the assembly. Benchmarking Universal Single‐Copy Orthologs (BUSCO) (v5.2.2) (Seppey et al., 2019) with the embryophyta_odb10.2020–09‐10 database was used to evaluate the completeness of the assembly. WGS reads obtained via BGISeq were first used to estimate genome size and heterozygosity with Jellyfish (v2.26) (Marçais & Kingsford, 2011) and GenomeScope (v1.0.0) (Ranallo‐Benavidez et al., 2020). These BGISeq WGS reads were also used to evaluate assembly completeness using BWA (v0.7.17) (Li, 2013) and SAMtools (v1.9) (Li et al., 2009).
2.5. Repeat and gene annotation
Transposable elements (TEs) and tandem repeats were annotated with the following workflows. TEs were identified via a combination of de novo and homology‐based approaches. We first customized a de novo repeat library for the genome via RepeatModeler (v1.0.11) (Price et al., 2005), which is based on two de novo repeat‐finding programs, RECON (v1.08) (Bao & Eddy, 2002) and RepeatScout (v1.06) (Benson, 1999). Long terminal repeat retrotransposons (LTR‐RTs) were subsequently identified via LTRharvest (v1.0) (Ellinghaus et al., 2008) and LTR_FINDER (Xu & Wang, 2007). High‐quality intact LTR‐RTs and nonredundant LTR libraries were subsequently obtained via LTR_retriever (v2.8) (Ou & Jiang, 2018). A nonredundant species‐specific TE library was constructed by combining the de novo TE sequences identified above with the publicly available TE sequences in the Repbase (v21.12) (Jurka et al., 2005), REXdb (Neumann et al., 2019), and Dfam databases. This nonredundant TE library was used to search against the entire genome sequence with RepeatMasker to identify fragmented TEs in the genome.
We integrated de novo, homology‐based, and transcript‐based predictions to annotate protein‐coding genes in the genome. The de novo gene models were predicted via two ab initio gene‐prediction software tools, namely, Augustus (v3.2.1) (Stanke et al., 2006) and SNAP (v2006‐07‐28) (Johnson et al., 2008). For the homology‐based approach, GeMoMa (Keilwagen et al., 2018) was generated via reference gene models from the plant order Apiales (to which Araliaceae plants, including Apium graveolens [Li et al., 2020], Aralia elata [Wang, Zhang, et al., 2022], Coriandrum sativum [Song et al., 2020], Daucus carota [Iorizzo et al., 2016], Eleutherococcus senticosus [Yang et al., 2021], and Panax ginseng [Wang, Wang, et al., 2022], belong). For transcript‐based prediction, RNA‐seq data were mapped to the reference genome using HISAT2 and assembled with StringTie. Gene models from these different approaches were combined via MAKER (v3.31.8) (Holt & Yandell, 2011). The final gene models were annotated by searching the GenBank Nonredundant, TrEMBL (Boeckmann et al., 2003), SwissProt (Boeckmann et al., 2003), eukaryotic orthologous group (KOG) (Koonin et al., 2004), Gene Ontology (GO) (Ashburner et al., 2000), InterPro (Hunter et al., 2009), and Kyoto Encyclopedia of Genes and Genomes (KEGG) (Kanehisa & Goto, 2000) databases via BLASTP with a cutoff E‐value threshold of 10−5.
2.6. Gene families and phylogenetic analysis
OrthoFinder (v2.5.5) (Emms & Kelly, 2015) software was used to classify the protein sequences of the selected species into families. Comparative genomic analysis was performed on the three Polyscias genomes and 12 dicotyledonous species [Vitis vinifera L., Populus trichocarpa Torr. & A. Gray ex. Hook., Arabidopsis thaliana (L.) Henh., Theobroma cacao L., D. carota L., Angelica sinensis (Oliv.) Diels, Ap. graveolens L., Peucedanum praeruptorum Dunn, E. senticosus (Rupr. & Maxim.). Maxim., Ar. elata (Miq.). Seem., Pa. ginseng C.A. Mey, and Panax notoginseng (Burkill) F. H. Chen], with Oryza sativa L. as an outgroup species. A phylogenetic tree of 246 single‐copy gene sequences from the selected species was constructed using IQ‐Tree (v2.2.0) (Minh et al., 2020) software. First, MAFFT (v7.429) (Katoh et al., 2002) was used to align the sequences of each single‐copy gene family, followed by the use of PAL2NAL (v14) (Suyama et al., 2006) to convert protein sequence alignments to nucleotide sequence alignments. Next, Gblocks (Castresana, 2000) was used to remove regions with poor sequence alignment or large differences, and all well‐aligned gene families of each species were linked from end to end. Finally, IQ‐Tree ModelFinder was used to construct the phylogenetic tree using the maximum likelihood method with 1000 bootstrap replicates.
The divergence times of the 16 species were estimated via the r8s program v1.2 (Sanderson & Michael, 2003) and further confirmed via MCMCTree from the PAML package v4.6 (Ziheng, 2007). The molecular clock model and nucleotide substitution model for MCMCTree were set as the “correlated rates model” and “JC69” model, respectively. The Markov chain Monte Carlo (MCMC) analysis was run for 100,000 generations using a burn‐in of 10,000 iterations. The calibrations for the divergence times of the 16 species were obtained from the TimeTree database with upper and lower bounds (http://www.timetree.org/) (Hedges et al., 2006).
CAFE v4.0 (Bie et al., 2006) was used to determine the expansion and contraction of orthologous gene families from the selected species. CAFE analyses of the evolution of gene gain and loss were based on the stochastic birth and death model. The specific gene families of Po. cf. bisattenuata, Po. lallanii, and Po. macgillivrayi that had contracted and expanded significantly (p < 0.05) were selected for KEGG enrichment analysis and GO term enrichment analysis via Ontologizer (http://ontologizer.de/) (Bauer et al., 2008).
2.7. Whole‐genome duplication analyses
BLASTP (v2.2.26) was used to compare the protein sequences of the two species and identify homologous gene pairs on the basis of sequence similarity. Next, the homologous gene pairs were used to identify collinear blocks via MCScanXpython version (v1.1.18) (Wang et al., 2012). On the basis of the distribution of Ks values (the number of synonymous substitutions per synonymous site) of paralogous genes, we determined the whole‐genome duplication (WGD) events using wgd (Zwaenepoel & Van de Peer, 2018). Publicly available scripts were used to calculate the proportion of conversion mutations to 4dTv bases in each homologous gene pair, and the HKY substitution model was used for correction. The timing of WGD events was estimated via the equation T = K/2r (r = 6.38 × 10−9).
2.8. Identification of the TPS family genes
According to the characteristics of TPS protein sequences containing the conserved N‐terminal Terpene_synth N (PF01397) and C‐terminal Terpene_synth C (PF03936) domains, the two hidden Markov model files were downloaded from the Pfam website to perform Hmmsearch (Finn et al., 2011) on the protein data of the three species, and the default threshold of 10.0 was applied. All possible TPS genes could be covered in the initial search, and two sequence sets were finally obtained. The two sequence sets were merged to eliminate redundancy, and the resulting dataset of Hmmsearch was obtained. To improve the TPS dataset, the homologous sequence dataset was obtained by using known TPS protein sequences (Aubourg et al., 2002; Tai et al., 2020; Thibaud‐Nissen et al., 2009) and searching the protein libraries of three species via BLASTP comparison. The two obtained datasets were eliminated, and redundancy was eliminated. Pfam was used again to conduct a domain check on the combined dataset, and the genes without the conserved domains and incomplete genes less than 300 aa in length were removed to obtain the final TPS gene list. After BLASTP and Hmm searches (PF01397 and PF03936), phylogenetic trees were constructed via the maximum likelihood method in IQ‐TREE (v2.2.0) with 1000 bootstrap replicates.
The protein sequences of the identified barley genes and previously reported TPS genes (Aubourg et al., 2002; Tai et al., 2020; Thibaud‐Nissen et al., 2009) were employed to construct the phylogenetic tree. Multiple sequence alignment was performed using Phylip (v4.0) (Misener & Krawetz, 2000) software, and the MEGA‐X tool was used to construct a phylogenetic tree via the neighbor‐joining (NJ) method with 1000 bootstrap replications. We interpreted and visualized the phylogenetic trees via the online tool iTOL v6 (Letunic & Bork, 2021). The exon‒intron structures of these TPS genes were retrieved according to the genome annotation files. The conserved protein motifs were predicted via MEME (v5.5.1) (Bailey et al., 2009) tools with the following parameters: the maximum number of motifs was set to 10, any number of repetitions was allowed, and the optimum width ranged from 6 to 25. The 2.0‐kb genomic sequences upstream of the transcription start sites of the TPS genes were extracted from the barley reference sequence via an in‐home Perl script and submitted to the PlantCARE (Lescot et al., 2002) database to predict the supposed cis‐acting transcriptional regulatory elements.
2.9. RNA‐seq data analysis
Quality control of the raw reads was first performed via Trimmomatic, and the clean reads were subsequently aligned to the genome via HISAT2 (v2.1.0) (Kim et al., 2015). Gene expression levels were estimated on the basis of fragments per kilobase of transcript per million fragments mapped via StringTie2 (v1.2.2) (Pertea et al., 2015). TBtools (v1.0987) (Chen et al., 2020) was used for data normalization via the z‐score method.
2.10. Infrageneric comparisons among Polyscias species
Syntenic blocks within and among the three genome assemblies were analyzed via MCScanX in the jcvi package and visualized via jcvi (v1.1.19) (Tang et al., 2008). The default parameters were used for the synteny analysis in MCScan, with the exception that the parameter “minimum number of anchors” was set to 10. MCScanX (match score 3 and match size 10) was also used, and the results were visualized.
2.11. Identification of metabolic gene clusters
The genomic sequence and annotation files of the three genomes were subsequently transferred to the PlantiSMASH (Kautsar et al., 2017) program with the parameters –input‐type nucl –taxon plant. Clusters of genes from at least two enzyme families collocated at the same locus were identified as metabolic clusters.
2.12. GC‒MS analysis and identification of mono‐ and sesquiterpenoids
The leaf and stem tissues of the eight Polyscias species studied herein were also collected for metabolic analysis. The air‐dried (AD) samples were dried at room temperature in the dark and then crushed to obtain a powder. Three different solvent extracts, hexane, dichloromethane/methanol (1:1, v/v), and methanol/water (4:1, v/v), were prepared and concentrated under nitrogen gas. This yielded the final extracts. One hundred microliters of this mixture were diluted in 900 µL of dichloromethane. Then, 100 µL of this dilution was transferred to a headspace vial, and 20 µL (10 µg/mL) of internal standard solution was added. The samples were analyzed via fully automated headspace solid‐phase microextraction (HS‐SPME) followed by gas chromatography‒mass spectrometry (GC‒MS) analysis. After sampling, desorption of the VOCs (volatile organic compounds) from the fiber coating was performed in the injection port of the GC‒MS instrument (model 8890; Agilent) at 250°C for 5 min in splitless mode. The identification and quantification of VOCs were performed via an Agilent 8890 GC and a 7000D mass spectrometer (Agilent) equipped with a 30 m × 0.25 mm × 0.25 µm DB‐5MS (5% phenyl‐polymethylsiloxane) capillary column, and the area under the curve was adjusted to the internal standard. Helium was used as the carrier gas at a linear velocity of 1.2 mL/min. The injector temperature was maintained at 250°C. The oven temperature program started from 40°C (3.5 min), followed by an increase at 10°C/min to 100°C, at 7°C/min to 180°C, and at 25°C/min to 280°C, where it was held for 5 min. Mass spectra were recorded in electron impact (EI) ionization mode at 70 eV. The quadrupole mass detector, ion source, and transfer line temperatures were set at 150, 230, and 280°C, respectively. MS in selected ion monitoring (SIM) mode was used for the identification and quantification of analytes. Chemical profiling was performed in triplicate, and three biological replicates were examined. The raw data were analyzed via Agilent MassHunter Quantitative Analysis. Signals with aligned (same RT range and similar peak shape) quantitative ion peaks and qualitative ion peaks were considered detected. These signals were annotated according to the MS2T library, and peak areas were integrated and manually adjusted (Yuan et al., 2022). Following identification, the list of identified compounds was sorted to include only compounds with an area of at least 100k. The structure was identified along with the name, which was checked against the PubChem (https://pubchem.ncbi.nlm.nih.gov/) and PlantaEdb (https://plantaedb.com/) databases. Duplicates were removed. Compounds from the genus Polyscias that were previously identified and isolated were investigated herein.
3. RESULTS
3.1. Genome assembly and annotation
Following plant collection on Kauaʻi, DNA was extracted from the collected samples, and a genome analysis was performed for Po. cf. bisattenuata, Po. kavaiensis, Po. macgillivrayi, Po. oahuensis, Po. lallanii, Po. sandwicensis, Po. waialealae, and Po. waimeae. Whole‐genome sequences were obtained for only Po. cf. bisattenuata, Po. lallanii, and Po. macgillivrayi as representative species with long‐read sequence data for assembly (Table 1). A total of 36.2, 31.6, and 30.5 Gb of long reads were generated via the PacBio platform (Tables S3–S5). De novo assembly of these CCS reads from Po. cf. bisattenuata generated an initial genome assembly of 2.03 Gb consisting of 714 contigs with an N50 length of 12.1 Mb (Tables 1 and S6). The size of the Po. lallanii draft genome was 1.86 Gb, including 4406 contigs with an N50 length of 8.64 Mb (Table S7). The resulting Po. macgillivrayi assembly was 954 Mb in length and contained 1148 contigs (N50 44.7 Mb) (Table S8). The assembly results were evaluated via BUSCO analysis, which revealed that 98.7%, 98.9%, and 98.7% of the 1614 core conserved genes were present in Po. cf. bisattenuata, Po. lallanii, and Po. macgillivrayi, respectively (Tables S9 and S10; Figure S1). The genomes of Po. cf. bisattenuata and Po. lallanii are predicted to be tetraploid, whereas those of Po. macgillivrayi are diploid.
TABLE 1.
Assembly and annotation statistics of the three genomes.
| Polyscias cf. bisattenuata | Polyscias lallanii | Polyscias macgillivrayi | |
|---|---|---|---|
| Genome size | 2,036,654,447 | 1,860,474,263 | 954,059,996 |
| Contig N50 | 12,158,567 | 8,642,933 | 44,703,306 |
| Contig L50 | 47 | 57 | 7 |
| Contig N90 | 2,027,060 | 607,404 | 6,120,914 |
| Contig L90 | 184 | 299 | 26 |
| GC content (%) | 33.2 | 36.5 | 36.7 |
| Genome BUSCO (%) | 98.7 | 98.9 | 98.7 |
| Gene number | 51,083 | 60,881 | 29,060 |
| Gene BUSCO (%) | 99.0 | 99.5 | 98.7 |
Abbreviation: BUSCO, Benchmarking Universal Single‐Copy Orthologs.
Transposal elements (TE) are found in large proportions in the genomes of eukaryotic species. A total of 57.92% of the Po. cf. bisattenuata genome was annotated as repetitive elements, with corresponding values of 46.34% for the Po. lallanii genome and 73.54% for the Po. macgillivrayi genome (Table S11; Figures S2 and S3). Protein‐coding genes were predicted via homology, ab initio, and transcriptomic prediction analysis. In total, 51,083 genes were predicted in the Po. cf. bisattenuata genome, with an average gene length of 5408 bp and an average of six exons per gene. A total of 60,881 genes were predicted in the Po. lallanii genome, with an average gene length of 4554 bp, and 29,058 genes were predicted in the Po. macgillivrayi genome (Table 1; Figure S4). BUSCO analysis revealed that the core conserved genes accounted for 99.0%, 99.5%, and 98.7% of the predicted genes in Po. cf. bisattenuata, Po. lallanii, and Po. macgillivrayi (Table S12), respectively, indicating the high quality of the gene annotation (Figures S5 and S6).
3.2. Evolutionary analysis of Polyscias species
A total of 32,682 gene families were identified in the comparative genomic analysis, including 7031 that were common among all 16 species (Figures S7 and S8; Table S13). We extracted 246 single‐copy orthologous genes derived from the single‐copy gene family analysis and then used the Bayesian relaxation molecular clock method to estimate the divergence times. The three Polyscias species were clustered most closely, with a divergence time of approximately 26.6 million years (Mya) (Figure 1A). Among the Po. cf. bisattenuata and Po. lallanii genes, 5525 had significantly expanded gene families, and they presented the smallest Ks peaks, which indicated a recent polyploidization event (Figure 1C; Table S14). In addition, a comparative analysis of the gene families of four Araliaceae species (Po. cf. bisattenuata, Po. lallanii, Po. macgillivrayi, and Pa. ginseng) with those of A. thaliana demonstrated that the genus Polyscias shared more gene families with Pa. ginseng (1572) than A. thaliana (275) (Figure 1D), demonstrating a close relationship between Polyscias and Pa. ginseng. The GO and KEGG enrichment results revealed that the enriched gene families in the three genomes are involved in terpenoid biosynthetic pathways (Figures S9 and S10; Tables S15–S17).
FIGURE 1.
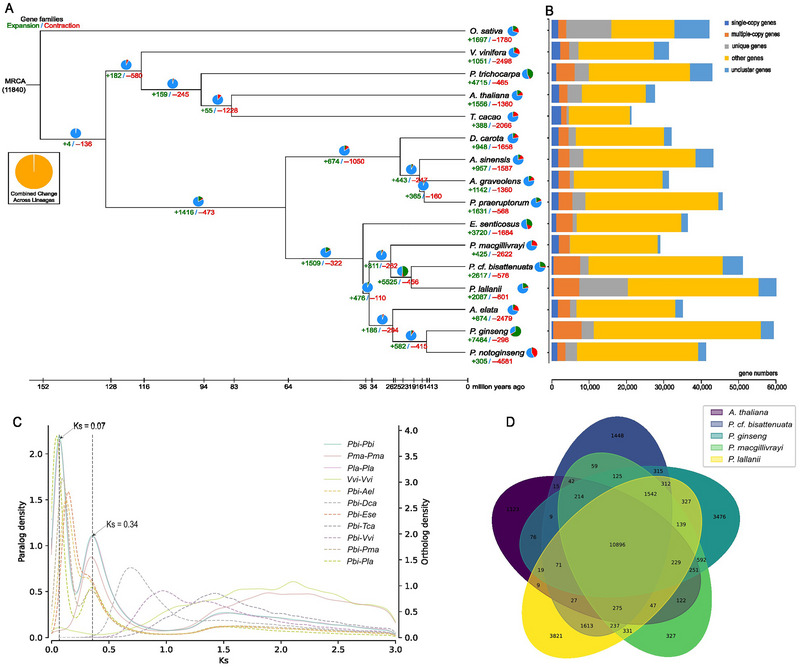
Evolutionary and annotation analyses of Polyscias cf. bisattenuata, Polyscias lallanii, and Polyscias macgillivrayi. (A) Inferred phylogenetic tree. Gene family expansions are indicated in green, and gene family comparisons are indicated in red. (B) Gene family analysis results. (C) Distributions of synonymous substitutions per synonymous site (Ks) of one‐to‐one orthologs identified among Po. cf. bisattenuata, Po. lallanii, Po. macgillivrayi, Aralia elata, Daucus carota, Eleutherococcus senticosus, Theobroma cacao, and Vitis vinifera. (D) Venn diagram of the gene families of the five species (Po. cf. bisattenuata, Po. lallanii, Po. macgillivrayi, Panax ginseng, and Arabidopsis thaliana).
3.3. Identification of genes in the TPS gene family
TPSs are key enzymes in the biosynthesis of smaller terpenoids and often perform the first step in the biosynthesis of individual terpenes. TPSs are widely found in members of the order Apiales, including Polyscias. We systematically characterized the TPS gene family members in the three Polyscias genomes (Po. cf. bisattenuata, Po. macgillivrayi, and Po. lallanii) along with five additional transcriptomes (Po. kavaiensis, Po. oahuensis, Po. sandwicensis, Po. waialealae, and Po. waimeae). This was compared with the presence of TPSs in the Araliaceae species Panax quinquefolius, Pa. notoginseng, Pa. ginseng, Panax stipuleanatus, Panax japonicus, and Ar. elata on the basis of the HMM scan and BLASTP search results. In Po. cf. bisattenuata, 38 TPS genes were identified; in Po. lallanii and Po. macgillivrayi, we identified 43 TPS genes and 26 TPS genes, respectively. All the genes encoding proteins were more than 300 aa in length and contained the complete PF011397 and PF03936 domains. The key information for all the TPS genes is shown in Table 2. These TPS genes provide the basis for our subsequent in‐depth study of their roles in the formation of terpenoids.
TABLE 2.
Annotation of TPS subfamilies in Araliaceae species.
| Species | Total | TPS‐a | TPS‐b | TPS‐c | TPS‐d | TPS‐e/f | TPS‐g |
|---|---|---|---|---|---|---|---|
| Polyscias cf. bisattenuata | 38 | 7 | 16 | 7 | 0 | 5 | 3 |
| Polyscias kavaiensis | 26 | 6 | 3 | 4 | 0 | 6 | 7 |
| Polyscias macgillivrayi | 26 | 10 | 12 | 1 | 0 | 2 | 1 |
| Polyscias oahuensis | 15 | 3 | 4 | 2 | 0 | 3 | 3 |
| Polyscias lallanii | 43 | 9 | 20 | 8 | 0 | 3 | 3 |
| Polyscias sandwicensis | 14 | 1 | 4 | 0 | 0 | 1 | 8 |
| Polyscias waialealae | 13 | 4 | 4 | 2 | 0 | 2 | 1 |
| Polyscias waimeae | 19 | 6 | 2 | 1 | 0 | 2 | 8 |
| Aralia elata | 63 | 26 | 22 | 5 | 0 | 1 | 9 |
| Panax ginseng | 54 | 17 | 18 | 6 | 0 | 5 | 8 |
| Panax japonicus | 48 | 18 | 13 | 5 | 0 | 6 | 6 |
| Panax notoginseng | 21 | 6 | 7 | 3 | 0 | 2 | 3 |
| Panax quinquefolius | 39 | 11 | 16 | 3 | 0 | 6 | 3 |
| Panax stipuleanatus | 30 | 5 | 10 | 3 | 0 | 3 | 9 |
On the basis of protein domain and phylogenetic analyses, we explored the subfamilies of the TPS family. To identify information about the subfamily placement of the TPS genes, we used 89 definite genes representing the TPS subfamilies from previous studies (Aubourg et al., 2002; Tai et al., 2020; Thibaud‐Nissen et al., 2009). The identified genes were divided into six subfamilies, which were labeled TPS‐a to TPS‐g, excluding TPS‐d (Figures S11–S15), as described previously. The TPS‐b subfamily had the most members among the three Polyscias genomes (Figures 2 and 3A). Since the analysis of gene structure facilitates the understanding of gene evolution and possible roles, the intron‒exon structure and conserved motifs of those TPS genes were analyzed. All the TPS genes possessed two conserved domains (PF011397 and PF03936) (Figure 3C). A total of 10 conserved motifs were also identified in these TPS genes. A majority of the TPS‐a, TPS‐b, and TPS‐g genes presented relatively consistent motif compositions, with eight to 10 conserved motifs each, whereas the TPS‐c, TPS‐e, and TPS‐f contained five to seven conserved motifs each (Figures 3B and FS16). These variations in conserved motif compositions might correlate with the different functions of these TPS genes.
FIGURE 2.

Phylogenetic analysis of 107 candidate terpene synthase (TPS) genes with 231 TPS genes from other genomes (Aubourg et al., 2002; Tai et al., 2020; Thibaud‐Nissen et al., 2009; Tables S18 and S19).
FIGURE 3.

Phylogenetic relationship, gene structure, and conserved motif analyses. (A) Phylogenetic analysis of the terpene synthase (TPS) genes in Polyscias cf. bisattenuata (Pbis), Polyscias lallanii (Plal), Polyscias macgillivrayi (Pmac), and Polyscias ginseng (GWHPBEIL). Members of different subfamilies are marked with different background colors. (B) Functional motif compositions of the TPS genes. The 10 motifs are represented by rectangular boxes of different colors. (C) The conserved functional domains of PF001397 and PF03936 in the TPS gene are represented by dark turquoise and light blue, respectively. (D) Intron‒exon organization of the TPS gene. The introns and exons are represented by the broken line and gray boxes, respectively. (E) The number and functional classification of cis‐acting elements in each TPS gene. (F) Distribution of 57 identified cis‐acting elements in each TPS gene. The elements are represented by boxes of different colors.
3.4. Regulatory elements in the TPS genes
The exon‒intron structure is an evolutionary force that determines the functional diversification of members of a gene family. The intron characteristics of the TPS genes were highly variable, with numbers ranging from 3 to 19. PbisTPS‐b11 and PbisTPS‐a4 had long introns, which might have been due to the insertion of retrotransposon‐like elements. These TPS genes also displayed typical intron‒exon structural characteristics; the TPS‐c, TPS‐e, and TPS‐f genes were distinguished by longer sequences and more introns than are found in other subfamilies (Figure 3D) (Yang et al., 2023).
A total of 57 kinds of cis‐acting elements were identified in the promoter sequences (in the regions 2000 bp from the transcription start sites) of the TPS genes. The elements were classified according to phytohormone responsiveness, plant growth and development, and stress responsiveness. The elements associated with biotic or abiotic stress responses were identified in almost all the TPS genes, especially those related to the light response (Box 4, G‐box) (Figure 4E,F). Similarly, almost all the TPS genes contained methyl jasmonate (MYC) and jasmonate ethylene responsive elements (EREs), which are often linked to both abiotic and biotic stress events in plant cells. These results indicate that hormones such as methyl jasmonate play a vital role in the initiation of terpenoid biosynthesis in Polyscias plants, which is often observed in plants (Feng et al., 2024; Martin et al., 2002).
FIGURE 4.

Evolution of terpene synthase (TPS) genes and biosynthesis pathways. (A, B) Synteny of the Polyscias cf. bisattenuata, Polyscias lallanii, and Polyscias macgillivrayi genes in the TPS‐b gene cluster. (C) Synteny of the Po. cf. bisattenuata, Po. lallanii, and Po. macgillivrayi genes in the TPS‐a gene cluster. (D) Terpene biosynthesis gene clusters (BGCs) in Po. macgillivrayi and gene synteny with Po. cf. bisattenuata and Po. lallanii.
3.5. Analysis of the TPS‐b gene family
Significant expansion and retention of the TPS‐b subfamily were observed in Polyscias plants (Figure 2; Table 2). Thus, a localization analysis was performed, and it revealed that the Po. macgillivrayi genome had two TPS‐b gene clusters located within the 19.90–20.08 Mb region on contig4 (Figure 4A) and the 71.88–71.94 Mb region on contig8 (Figure 4B). Contig4 contained five repeat modules of the TPS‐b genes, all of which were on the plus strand. On contig8, there were three repeated TPS‐b genes on the plus strand and two on the minus strand, which might have resulted from a duplication event because of their close relationship (Hanada et al., 2008; Wang et al., 2021).
Intergenomic syntenic comparison was performed of the two Po. macgillivrayi gene clusters with those of 11 other species (Po. cf. bisattenuata, Pa. quinquefolius, Pa. notoginseng, Pa. ginseng, Pa. stipuleanatus, Pa. japonicus, Ar. elata, Po. lallanii, A. sinensis, Pe. praeruptorum, and D. carota) (Figure S17). Homologous regions were observed of these two gene clusters in Po. cf. bisattenuata and Po. lallanii, which contain two homologous copy regions. Polyscias lallanii and Po. cf. bisattenuata were compared with Po. macgillivrayi (Figure 4A,B), suggesting that tandem duplication occurred within the TPS family of Polyscias plants, especially in the TPS‐b subfamily. This phenomenon is consistent with the results for other reported genomes (Chaw et al., 2019; Wang, Zhang, et al., 2022; Yang et al., 2023).
Polyscias cf. bisattenuata contained one TPS‐a gene cluster, which was located within the 1.36–1.95 Mb region on contigA65 (Figure 4C). There were four repeat modules of the TPS genes, all of which were on the plus strand. We compared this region with those of other species via comparative homology analysis. Only three closely packed TPS‐a genes were found in the homologous region of Po. macgillivrayi. Polyscias lallanii does not have a TPS‐a gene cluster. Genes belonging to this cluster were not grouped together in their corresponding subfamily phylogeny (Figure S11), suggesting that their arrangement might have occurred more recently than the last WGD event.
3.6. Biosynthetic pathways of terpenoids
Two canonical pathways are involved in the biosynthesis of terpenes: the mevalonic acid (MVA) pathway in the cytosol for sesquiterpene and triterpene biosynthesis and the methylerythritol phosphate (MEP) pathway in plastids for monoterpene, diterpene, and tetraterpene biosynthesis. In this study, the genes involved in both pathways were identified, and their expression levels were evaluated and compared among leaves, stems, and flowers. Almost all the genes were expressed at the highest levels in flowers, followed by leaves, and the lowest levels were detected in stems (Figure 5).
FIGURE 5.

Expression profiles of genes encoding enzymes involved in canonical terpenoid biosynthesis pathways. Abbreviations for enzymes in each catalytic step are shown in red, and their full names are listed in Table S20.
TPSs are the key enzymes responsible for the biosynthesis of terpenoid compounds (Nagegowda & Gupta, 2020). In this study, most of the TPS‐b genes were annotated as possible monoterpene synthases, such as putative 1,8‐cineole synthases (Pichersky & Raguso, 2018; Zapata & Fine, 2013); two of the TPS gene clusters were involved in putative 1,8‐cineole biosynthesis and alpha‐terpineol biosynthesis, but these genes presented relatively low expression levels. Most TPS‐a genes are generally involved in mono‐ or sesquiterpenoid biosynthesis, and for the putative germacrene D synthases, all three genomes had two TPS‐a genes with high expression levels.
We identified diverse specialized metabolite biosynthesis gene clusters (BGCs) in the three Polyscias genomes. BGCs include genes that are nonrandomly ordered on chromosomes and may optimize the synthesis of natural products in living organisms (Smit & Lichman, 2022; Yan et al., 2023). Polyscias cf. bisattenuata contained 30 possible BGCs, the largest number of BGCs in the three Polyscias genomes. There were many terpene BGCs in the three Polyscias genomes. The genes of one of the terpenoid BCGs are shown in Figure 4D. Our findings indicate that BGCs are not conserved across Polyscias. Substantial gains and losses of BGCs and genes were observed (Table 3).
TABLE 3.
Identification of possible biosynthesis‐related gene clusters (BGCs).
| BGCs | Terpene | Saccharide | Polyketide |
|---|---|---|---|
| Polyscias cf. bisattenuata | 4 | 14 | 6 |
| Polyscias lallanii | 5 | 11 | 2 |
| Polyscias macgillivrayi | 2 | 13 | 3 |
| Peucedanum praeruptorum | 5 | 11 | 3 |
| Aralia elata | 4 | 6 | 0 |
| Panax ginseng | 17 | 21 | 7 |
| Panax japonicus | 14 | 22 | 4 |
| Panax notoginseng | 5 | 10 | 2 |
| Panax quinquefolius | 9 | 17 | 3 |
| Panax stipuleanatus | 5 | 11 | 3 |
3.7. Identification of mono‐ and sesquiterpenoids
As shown in Table 4, 107 mono‐ and sesquiterpenoids were identified in the leaves and stems of the eight studied Polyscias species. The chemical contents of these eight species have not been previously studied, and this analysis was only qualitative. Some of the compounds, such as artemisia alcohol and menthone, are present in very high amounts in all species, whereas others are exclusively present in only one species, such as hinesol. Some of the identified compounds have been found in other Polyscias species, indicating that a common TPS gene is shared across the genus.
TABLE 4.
Overview of the identified mono‐ and sesquiterpenoids of the investigated Polyscias species.
| Polyscias sandwicensis | Polyscias waialealae | Polyscias waimeae | Polyscias kavaiensis | Polyscias oahuensis | Polyscias cf. bisattenuata | Polyscias macgillivrayi | Polyscias lallanii | Found in Polyscias | References | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound name | Leaves | Stem | Leaves | Stem | Leaves | Stem | Leaves | Stem | Leaves | Stem | Leaves | Stem | Leaves | Stem | Leaves | Stem | ||
| 2‐Hydroxypiperitone | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 476,592 | 267,709 | 256,089 | 383,689 | 457,985 | 393,911 | ||
| 2‐Methylisoborneol | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 2,338,495 | 1,564,707 | 1,431,494 | 2,180,838 | 2,298,086 | 2,204,413 | ||
| 4,11‐Selinadiene | 386,632 | 183,046 | 2,022,828 | 498,991 | 1,487,049 | 169,981 | 875,136 | 208,597 | 868,529 | 1,032,163 | 8,293,691 | 854,962 | 3,619,747 | 572,453 | 5,149,933 | 2,543,280 | ||
| 6,9‐Guaiadene | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 554,934 | n.d. | 216,735 | n.d. | 578,491 | n.d. | P. guilfoylei | https://doi.org/10.2174/2210315509666190624103355 |
| 6‐Epishyobunone | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 290,105 | n.d. | 183,130 | n.d. | 307,238 | 154,957 | ||
| 8‐Hydroxylinalool | n.d. | n.d. | 239,683 | 155,116 | 205,449 | 190,390 | 101,149 | n.d. | 115,755 | 106,758 | 1,357,511 | 791,868 | 725,284 | 1,197,123 | 1,259,285 | 1,202,980 | ||
| Acoradiene | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 6,218,459 | 282,666 | 183,046 | n.d. | 1,288,900 | 655,649 | ||
| α‐Bergamotene | 422,930 | 440,924 | 1,158,035 | 421,483 | 1,844,979 | 2,479,841 | 3,600,680 | 4,455,188 | 635,027 | 157,442 | 2,190,000 | 350,631 | 2,029,500 | 407,103 | 4,751,157 | 1,993,580 | P. fruticosa | https://doi.org/10.1002/ffj.2730050309 |
| α‐Bisabolene | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 6,508,059 | 335,620 | 596,033 | 220,760 | 2,359,122 | 1,014,743 | P. fruticosa, P. balfouriana, P. guilfoylei | |
| α‐Bulnesene | 289,715 | 180,789 | 9,636,100 | n.d. | 151,380 | n.d. | 156,505 | n.d. | 11,734,967 | 134,016 | 7,373,121 | 960,136 | 736,248 | 202,736 | 2,287,786 | 1,079,098 | ||
| α‐Cadinene | 200,678 | 210,972 | 825,768 | n.d. | 612,334 | 234,780 | 1,098,940 | 315,021 | 1,906,536 | 118,271 | 306,602 | 107,795 | 480,199 | n.d. | 466,197 | 122,592 | P. guilfoylei | https://doi.org/10.2174/2210315509666190624103355 |
| α‐Cubebene | 329,265 | 534,352 | 1,422,861 | 119,773 | 1,410,956 | 504,478 | 2,953,961 | 1,084,221 | 487,597 | n.d. | 1,503,839 | 138,422 | 1,177,396 | 195,007 | 4,338,200 | 1,036,575 | P. fruticosa, P. guilfoylei | |
| α‐Cuprenene | 320,939 | 111,015 | 5,912,363 | n.d. | n.d. | n.d. | 110,733 | n.d. | 7,011,789 | n.d. | 6,911,553 | 913,511 | 606,258 | 228,492 | 2,272,056 | 901,518 | ||
| α‐Eudesmol | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 279,643 | 379,276 | 573,509 | 486,623 | 1,877,722 | 126,489 | P. guilfoylei | https://doi.org/10.2174/2210315509666190624103355 |
| α‐Farnesene | 128,980 | 271,588 | 110,039 | 244,489 | 341,358 | 456,234 | 214,015 | 758,579 | 1,647,453 | 252,061 | 2,103,570 | 1,413,597 | 1,631,203 | 2,036,210 | 1,762,596 | 1,599,289 | ||
| α‐Gurjunene | 119,528 | 149,947 | 641,285 | n.d. | 450,424 | 150,174 | 1,098,668 | 376,681 | 176,065 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | ||
| α‐Muurolene | 225,962 | 111,984 | 1,350,263 | 349,389 | 605,369 | n.d. | 567,279 | 103,071 | 397,814 | 666,722 | 4,248,756 | 518,130 | 3,129,061 | 658,642 | 5,062,300 | 2,798,816 | ||
| α‐Pinene oxide | 171,280 | 180,981 | 139,642 | 178,136 | 176,615 | 111,774 | 140,302 | 162,635 | 150,957 | n.d. | 1,293,100 | 936,464 | 895,371 | 1,176,561 | 1,265,781 | 1,190,790 | ||
| α‐Sesquiphellandrene | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 2,384,693 | 263,963 | 1,497,330 | 353,538 | 2,788,687 | 1,485,709 | ||
| Aristolene | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 482,108 | 142,902 | n.d. | n.d. | n.d. | n.d. | ||
| Aristolochene | 341,833 | 206,756 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 117,556 | n.d. | 10,446,364 | 1,156,137 | 435,240 | 179,081 | 2,439,808 | 1,244,442 | P. guilfoylei | https://doi.org/10.2174/2210315509666190624103355 |
| Aromadendrene | n.d. | n.d. | 549,255 | n.d. | 290,574 | 129,642 | 102,045 | 215,143 | 245,836 | n.d. | n.d. | n.d. | 1,268,229 | n.d. | n.d. | n.d. | ||
| Artemisia alcohol | 1,796,224 | 2,441,186 | 2,137,491 | 2,291,771 | 2,253,320 | 1,591,472 | 1,652,884 | 2,318,874 | 2,537,020 | 1,323,685 | 17,574,504 | 12,287,148 | 11,342,722 | 15,495,520 | 17,399,137 | 15,310,011 | ||
| Artemisia ketone | 381,264 | 447,891 | 375,700 | 443,306 | 474,893 | 320,175 | 290,162 | 400,509 | 394,977 | 258,092 | 2,752,463 | 1,919,956 | 1,783,048 | 2,352,489 | 2,802,933 | 2,565,610 | ||
| Ascaridole | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 510,669 | 349,887 | 299,346 | 455,651 | 479,638 | 454,717 | ||
| β‐Bergamotene | 112,612 | 310,007 | n.d. | 195,759 | 252,778 | 479,191 | 173,512 | 471,661 | 174,950 | 200,969 | 1,561,331 | 1,121,103 | 1,022,845 | 1,779,942 | 1,514,108 | 1,309,471 | P. fruticosa | https://doi.org/10.1002/ffj.2730050309 |
| β‐Bourbonene | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 124,260 | n.d. | 537,184 | n.d. | ||
| β ‐Cadinene | 280,429 | 275,790 | 892,076 | 219,872 | 1,191,687 | 1,716,485 | 2,325,954 | 2,871,803 | 737,091 | n.d. | 22,279,541 | 912,310 | 1,792,539 | 740,689 | 7,132,901 | 2,664,932 | ||
| β‐Calacorene | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 2,167,143 | n.d. | 627,918 | 230,300 | 2,189,196 | 338,840 | P. fruticosa | https://doi.org/10.1002/ffj.2730050309 |
| β‐Caryophyllene | 262,351 | 283,268 | 1,106,015 | 437,796 | 1,576,397 | 2,517,963 | 3,656,031 | 4,523,675 | 597,895 | 106,939 | 2,401,661 | 1,338,183 | 1,805,792 | 1,738,953 | 1,557,956 | 1,159,540 | P. fruticosa | https://doi.org/10.1002/ffj.2730050309 |
| β‐Cubebene | 199,445 | 240,274 | 793,903 | n.d. | 664,104 | 302,842 | 1,259,876 | 370,861 | 1,494,281 | n.d. | 2,292,867 | 248,494 | 959,357 | 167,126 | 2,112,401 | 484,072 | P. fruticosa | https://doi.org/10.1002/ffj.2730050309 |
| β‐Elemene | 201,642 | 194,832 | 334,339 | n.d. | 183,085 | n.d. | 311,756 | 115,671 | 244,149 | n.d. | 4,835,464 | 458,890 | 255,277 | 195,519 | 1,734,928 | 535,828 | P. guilfoylei | https://doi.org/10.2174/2210315509666190624103355 |
| β‐Farnesene | n.d. | n.d. | 264,120 | n.d. | 404,803 | 585,169 | 784,701 | 893,064 | 125,299 | n.d. | 189,210 | n.d. | 120,058 | 114,933 | n.d. | n.d. | ||
| β‐Guaiene | 229,701 | 108,167 | 1,981,208 | 370,372 | 761,949 | 101,387 | 596,413 | 104,473 | 3,012,777 | 805,738 | n.d. | n.d. | 465,381 | n.d. | n.d. | n.d. | P. fruticosa, P. balfouriana | |
| β‐Gurjunene | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 2,107,104 | n.d. | 1,349,163 | 222,369 | 3,866,901 | 1,168,168 | ||
| β‐Himachalene | 154,323 | n.d. | 1,151,236 | 291,880 | 533,876 | n.d. | 492,040 | n.d. | 2,026,619 | 512,217 | n.d. | n.d. | 332,197 | n.d. | n.d. | n.d. | ||
| β‐Ionone epoxide | 349,918 | 194,201 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 10,221,589 | 1,108,049 | 429,293 | 172,711 | 2,259,367 | 1,105,725 | ||
| β‐Maaliene | 108,627 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 137,636 | n.d. | 3,429,486 | 247,781 | 144,711 | n.d. | 876,487 | 249,875 | ||
| β‐Ocimene | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 5,671,596 | 3,679,801 | 3,526,812 | 5,148,377 | 5,469,995 | 4,969,637 | ||
| β‐Phellandrene | 567,447 | 775,451 | 650,669 | 729,945 | 763,884 | 538,006 | 441,763 | 630,874 | 597,742 | 387,954 | n.d. | 138,838 | 259,713 | n.d. | n.d. | n.d. | ||
| β‐Pinene | 229,276 | 327,522 | 243,694 | 310,891 | 329,248 | 194,567 | 167,291 | 274,824 | 283,614 | 148,505 | 1,120,449 | 836,672 | 782,668 | 1,033,748 | 1,065,645 | 949,143 | ||
| β‐Santalene | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 6,174,065 | 5,772,457 | 7,575,281 | 7,817,536 | 3,659,339 | 2,716,553 | P. guilfoylei | https://doi.org/10.2174/2210315509666190624103355 |
| β‐Selinene | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 718,512 | 453,209 | 416,418 | 725,304 | 998,155 | 567,031 | ||
| β‐Sesquiphellandrene | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 1,259,702 | 863,119 | 818,244 | 1,323,119 | 1,295,954 | 920,270 | ||
| β‐Vetispirene | n.d. | 371,487 | 177,521 | 294,287 | 402,931 | 346,407 | 264,634 | 589,327 | 357,694 | 274,319 | 1,256,927 | 123,121 | 931,402 | 168,774 | 1,260,157 | 654,942 | ||
| Bicyclogermacrene | 262,846 | 112,150 | 1,361,037 | 334,608 | 833,992 | n.d. | 581,118 | 113,772 | 2,915,373 | 688,023 | 3,171,437 | 445,471 | 2,478,076 | 468,272 | 3,671,965 | 1,930,763 | ||
| Borneol | 2,263,462 | 3,250,260 | 3,081,349 | 3,087,477 | 4,025,415 | 2,776,229 | 2,851,751 | 3,432,511 | 3,589,939 | 1,991,418 | n.d. | 985,476 | 1,331,898 | n.d. | n.d. | n.d. | ||
| Bulnesol | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 164,681 | n.d. | n.d. | n.d. | 422,297 | n.d. | ||
| Cadina‐3,5‐diene | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 529,695 | n.d. | 313,838 | n.d. | 441,766 | 129,837 | P. fruticosa | https://doi.org/10.1002/ffj.2730050309 |
| Calamenene | 901,245 | 1,013,995 | 2,098,800 | 361,274 | 2,857,420 | 970,908 | 3,813,237 | 1,482,276 | 2,186,247 | 196,219 | 4,391,527 | 266,115 | 1,551,217 | 766,131 | 8,323,632 | 2,212,558 | ||
| Carvone | 586,149 | 823,719 | 791,509 | 750,540 | 1,055,069 | 840,188 | 779,080 | 908,369 | 990,513 | 705,539 | n.d. | 196,886 | 372,331 | n.d. | n.d. | n.d. | ||
| Cedr‐9‐ene | n.d. | 218,491 | 1,802,181 | 114,093 | 513,705 | 243,756 | 1,938,453 | 725,395 | 226,401 | n.d. | 573,311 | n.d. | 857,757 | n.d. | 2,972,432 | 467,539 | P. guilfoylei | https://doi.org/10.2174/2210315509666190624103355 |
| Cedrene | 294,845 | 285,203 | 785,015 | n.d. | 451,700 | 142,885 | 869,464 | 283,703 | 311,761 | n.d. | 1,861,504 | 372,581 | 402,811 | 176,615 | 1,476,736 | 472,654 | ||
| Chrysanthenol | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 600,118 | 388,086 | 372,644 | 548,301 | 696,562 | 580,843 | ||
| Citronellyl tiglate | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 176,736 | 275,694 | 336,372 | 274,892 | 623,925 | 115,674 | ||
| Cubebol | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 749,257 | 688,163 | 876,215 | 714,006 | 504,439 | 496,040 | ||
| Cuparene | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 252,884 | n.d. | 390,114 | n.d. | n.d. | n.d. | 139,626 | n.d. | ||
| Curcumene | 111,770 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 223,578 | n.d. | n.d. | n.d. | n.d. | ||
| Cyperene | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 818,137 | n.d. | 346,029 | n.d. | 726,329 | 250,637 | ||
| δ‐Amorphene | 172,254 | 108,547 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 180,136 | n.d. | n.d. | 253,064 | 106,488 | n.d. | n.d. | n.d. | ||
| δ‐Bisabolene | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 365,232 | n.d. | n.d. | n.d. | 1,333,540 | n.d. | P. guilfoylei | https://doi.org/10.2174/2210315509666190624103355 |
| Dihydrocarvone | 132,338 | 155,505 | 143,563 | 153,813 | 175,412 | 129,888 | 113,086 | 138,361 | 158,158 | 116,880 | 753,787 | 544,604 | 504,890 | 684,166 | 759,661 | 713,124 | ||
| Epizonarene | 1,785,752 | 834,247 | 14,808,171 | 106,758 | 650,521 | 242,053 | 741,078 | 329,282 | 17,036,054 | 299,541 | 4,909,551 | 3,313,372 | 2,929,273 | 523,700 | 4,309,243 | 2,258,538 | ||
| Eremophilene | 272,227 | 117,022 | 1,341,635 | 342,199 | 841,057 | n.d. | 583,659 | 101,180 | 2,688,407 | 593,154 | 4,034,470 | 473,695 | 2,937,657 | 657,388 | 4,485,571 | 2,396,715 | P. balfouriana, P. guilfoylei | |
| Eucarvone | 1,285,933 | 1,861,256 | 1,916,145 | 1,794,572 | 2,445,280 | 1,992,353 | 1,786,531 | 2,143,139 | 2,393,282 | 1,762,731 | n.d. | 395,713 | 771,837 | n.d. | n.d. | n.d. | ||
| Eudesma‐2,4,11‐triene | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 854,922 | 102,854 | n.d. | n.d. | 201,398 | n.d. | ||
| Fenchone | 144,473 | 142,154 | 121,656 | 143,009 | 144,850 | n.d. | 105,225 | 132,031 | 120,910 | n.d. | 1,159,506 | 807,670 | 729,258 | 971,883 | 1,113,816 | 994,159 | ||
| γ‐Bisabolene | n.d. | n.d. | 304,737 | n.d. | n.d. | n.d. | 213,992 | n.d. | 981,873 | n.d. | 1,281,660 | 104,587 | 257,856 | n.d. | 658,225 | 224,296 | P. fruticosa | https://doi.org/10.1002/ffj.2730050309 |
| γ ‐Cadinene | n.d. | n.d. | 323,627 | n.d. | 232,736 | 114,047 | n.d. | 215,543 | 107,836 | n.d. | 5,734,154 | 252,130 | 188,971 | n.d. | 1,276,687 | 593,419 | P. balfouriana, P. guilfoylei | https://doi.org/10.4172/2167‐0412.1000321 |
| γ‐Caryophyllene | 121,705 | 131,240 | 251,961 | n.d. | 185,986 | 103,309 | 246,738 | 132,718 | 190,238 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | ||
| γ‐Geraniol | 687,447 | 1,024,080 | 977,023 | 922,970 | 1,171,523 | 830,213 | 746,096 | 987,900 | 1,087,500 | 749,682 | 5,893,906 | 4,170,348 | 3,897,541 | 5,366,631 | 5,971,597 | 5,593,655 | ||
| γ‐Gurjunene | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 978,485 | 334,642 | 1,683,676 | 440,719 | P. balfouriana, P. guilfoylei | |
| γ‐Selinene | 291,274 | 186,670 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 11,649,096 | 1,081,958 | 367,554 | 152,352 | 2,263,825 | 1,038,068 | ||
| Geraniol | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 1,219,882 | 683,664 | 573,661 | 1,028,187 | 1,307,198 | 1,088,970 | ||
| Germacrene D | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 907,544 | n.d. | 273,192 | 123,930 | 3,682,778 | 157,214 | P. fruticosa | https://doi.org/10.1002/ffj.2730050309 |
| Hinesol | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 517,501 | n.d. | ||
| Humulene | 102,708 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 6,247,044 | 1,303,069 | 1,784,701 | 1,197,663 | 2,160,514 | 1,178,256 | ||
| Ipsenol | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 737,892 | 533,365 | 486,155 | 663,718 | 728,493 | 651,761 | ||
| Iridomyrmecin | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 307,860 | 144,448 | 231,552 | n.d. | 617,177 | 154,602 | ||
| Irone | n.d. | n.d. | 188,390 | n.d. | n.d. | n.d. | n.d. | n.d. | 656,210 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | ||
| Isobornyl formate | 2,375,849 | 3,371,078 | 3,271,378 | 3,073,947 | 4,320,481 | 3,430,549 | 2,922,946 | 3,522,669 | 3,901,493 | 2,900,949 | n.d. | 660,435 | 1,442,708 | n.d. | n.d. | n.d. | ||
| Isoitalicene | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 147,963 | n.d. | n.d. | n.d. | 431,926 | n.d. | ||
| Isolongifolene | 1,141,699 | 1,264,249 | 5,585,549 | 338,962 | 3,412,776 | 1,323,357 | 7,848,838 | 2,727,115 | 2,021,850 | 213,521 | n.d. | 926,441 | 1,026,816 | n.d. | n.d. | n.d. | ||
| Isopiperitenol | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 483,430 | 272,915 | 235,557 | 404,366 | 572,105 | 427,431 | ||
| Limonene‐1,2‐diol | 148,051 | 425,022 | 864,507 | 390,814 | 604,432 | 497,103 | 334,595 | 431,571 | 411,544 | 480,368 | 806,763 | 523,434 | 593,294 | 664,418 | 766,655 | 701,212 | ||
| Linalyl acetate | 376,256 | 521,870 | 539,900 | 539,938 | 680,612 | 560,293 | 545,804 | 594,116 | 665,979 | 490,962 | 3,377,946 | 2,427,842 | 2,256,404 | 3,020,347 | 3,193,667 | 3,053,165 | ||
| Menthone | 1,259,470 | 1,745,226 | 1,513,144 | 1,607,554 | 2,052,152 | 1,512,365 | 1,526,395 | 1,682,987 | 1,850,533 | 1,327,166 | 10,599,038 | 7,070,923 | 6,386,120 | 9,831,155 | 10,289,288 | 10,009,274 | ||
| Muurola‐4(14),5‐diene | 301,045 | 152,023 | n.d. | n.d. | n.d. | n.d. | 115,882 | n.d. | 428,268 | n.d. | n.d. | n.d. | 572,295 | n.d. | n.d. | n.d. | ||
| Neral | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 491,808 | 263,489 | 208,537 | 399,058 | 519,224 | 437,857 | ||
| Perillaldehyde | 519,529 | 759,895 | 644,507 | 634,241 | 872,247 | 550,379 | 557,000 | 733,930 | 807,422 | 506,077 | 125,454 | 233,437 | 320,708 | n.d. | 173,933 | 121,511 | ||
| Perillene | 423,397 | 462,833 | 363,512 | 452,333 | 430,722 | 275,236 | 334,824 | 417,001 | 394,740 | 265,011 | n.d. | 123,043 | 134,400 | n.d. | n.d. | n.d. | ||
| Pinocamphone | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 521,594 | 337,252 | 301,475 | 459,852 | 621,928 | 498,414 | ||
| Pinocarveol | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 429,263 | 243,103 | 192,775 | 346,983 | 454,104 | 391,338 | ||
| p‐Menthan‐1‐ol | 285,336 | 225,258 | 258,318 | 216,122 | 343,392 | 248,879 | 237,632 | 305,052 | 191,844 | 147,710 | 5,031,919 | 3,600,119 | 3,398,780 | 4,961,929 | 4,714,655 | 4,330,339 | ||
| Prezizaene | 491,596 | 511,423 | 1,690,313 | 579,025 | 2,315,689 | 3,215,818 | 4,348,077 | 5,274,823 | 726,075 | 174,208 | n.d. | n.d. | 288,950 | n.d. | n.d. | n.d. | ||
| Rose oxide | 221,080 | 224,387 | 193,873 | 247,529 | 246,565 | 159,106 | 143,777 | 207,508 | 213,570 | 131,317 | 1,538,762 | 1,028,018 | 918,226 | 1,329,345 | 1,498,482 | 1,335,402 | ||
| Salvial‐4(14)‐en‐1‐one | n.d. | n.d. | 285,815 | n.d. | 503,633 | 265,905 | 229,295 | 714,522 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 230,153 | n.d. | P. guilfoylei | https://doi.org/10.2174/2210315509666190624103355 |
| Sativene | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 4,039,076 | 744,255 | 404,125 | 1,072,239 | 1,303,959 | 511,135 | ||
| Selina‐3,7(11)‐diene | 744,946 | 639,212 | 3,196,732 | 217,200 | 1,608,694 | 562,692 | 2,903,851 | 907,982 | 8,591,341 | 536,310 | n.d. | 473,308 | 955,148 | n.d. | n.d. | n.d. | P. guilfoylei | https://doi.org/10.2174/2210315509666190624103355 |
| Selina‐5,11‐diene | 159,779 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 159,971 | 311,664 | n.d. | n.d. | n.d. | n.d. | ||
| Sesquirosefuran | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 416,310 | 313,386 | 276,439 | 471,185 | 345,379 | 323,143 | ||
| Thujone | 116,006 | 122,056 | n.d. | 124,392 | 125,134 | n.d. | n.d. | 111,495 | 108,480 | n.d. | 793,496 | 569,956 | 534,794 | 694,500 | 773,079 | 706,612 | ||
| Umbellulone | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 207,653 | 116,596 | 108,861 | 158,462 | 396,906 | 188,380 | ||
| Valerena‐4,7(11)‐diene | 126,869 | 108,111 | 135,633 | n.d. | 225,475 | 364,011 | 446,485 | 570,712 | n.d. | n.d. | n.d. | 223,075 | 214,618 | n.d. | 277,463 | n.d. | ||
| Viridiflorol | n.d. | n.d. | 490,859 | n.d. | 728,711 | 343,245 | 344,135 | 982,038 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 292,108 | n.d. | ||
| Ylangene | n.d. | 185,732 | 1,487,144 | 119,728 | 469,101 | 257,105 | 1,947,849 | 604,133 | 149,281 | n.d. | n.d. | n.d. | 127,007 | n.d. | n.d. | n.d. | P. balfouriana, P. guilfoylei | https://doi.org/10.4172/2167‐0412.1000321 |
Note: The numbers reflect the area under the curve, and the threshold for identification was set to 100k to be well clear of the minimum threshold of the instrument and for high‐confidence identification.
4. DISCUSSION
Three high‐quality reference genomes of Po. cf. bisattenuata, Po. lallanii, and Po. macgillivrayi are presented. WGD was identified, and the results indicated that the three species shared a common duplication event. The number of genes identified in the three species provides information about their ploidy, with Po. cf. bisattenuata and Po. lallanii being tetraploid and Po. macgillivrayi being diploid. This is quite common for closely related species with different ploidies. Polyploidy is a widespread evolutionary process and is particularly prevalent in plants (Barker et al., 2016; Husband et al., 2013; Soltis & Soltis, 2016). Our evolutionary analysis indicated that Po. cf. bisattenuata and Po. lallanii underwent one additional WGD event after the WGD that was shared with other species in Araliaceae. Polyploidy might lead to evolutionary advance, as duplicated genes acquire new functions (neofunctionalization), and polyploidy often leads to greater phenotypic and physiological plasticity (Hahn et al., 2012; Leitch & Leitch, 2008; Naghiloo & Vamosi, 2023). Previous studies have shown that polyploidy can have profound effects on plant genomes, phenotypes, and abiotic tolerance and is an important driver of ecological and evolutionary processes (Forrester et al., 2020; Yin et al., 2020).
The TPSs of the three species and closely related species were identified, and the phylogenetic and structural characteristics revealed that several TPSs were clustered and that these clusters might have been conserved during evolution. According to previous studies, the TPS gene family is a medium‐sized gene family. In this study, the diploid genome of Po. macgillivrayi consisted of 26 genes, whereas those of Po. cf. bisattenuata and Po. lallanii included 38 and 43 TPS genes, respectively. Genomic BUSCO evaluation indicated that the latter two species may have experienced gene loss after duplication (Ma et al., 2023), which is a well‐known phenomenon known as fractionation, wherein duplicated genes are lost over time (Cañestro et al., 2013). Following WGD, heavy loss of duplicated genes through fractionation often occurs, especially if the genes do not gain new functions. This serves energy for the plants, as large genomes are “energy” expensive to retain (Xiong et al., 2022). Different mechanisms of duplication can influence the fate of duplicated genes, and the loss of gene duplicates can impact the survival of the remaining paralogs (Xiong et al., 2022).
The number of TPS genes observed was consistent with previous observations for several other flowering plant species, as shown in Table 2. Larger gene clusters were observed in other Apiales species, consistent with the TPS gene clusters in Polyscias. Genes in the same cluster in a genome are often more homologous than other genes in the genome, which indicates that the gene cluster may have arisen through tandem duplication of genes (Xu et al., 2024; Yang et al., 2023). Moreover, the expression patterns of genes within the cluster were relatively consistent, indicating that they might share a nodal mechanism. Clustering of metabolism‐related genes is relatively conserved to ensure that common inheritance ensures that biosynthetic pathways are intact (Kautsar et al., 2017) while sharing similar regulatory mechanisms. TPS often colocalizes with cytochrome P450 genes (Boutanaev et al., 2015), but notably, in Polyscias species, we found only one gene cluster in which cytochrome P450 genes colocalized with TPS genes (He et al., 2022).
Terpene metabolism (monoterpenes, sesquiterpenes, and carotenoid biosynthetic enzymes) mostly exhibits rhythmic fluctuations at the transcriptional level (Zhou et al., 2015). Most of the upstream promoter regions of the TPS genes in the three species contained light‐responsive elements, such as light‐responsive element Box 4 (Yamada et al., 1994) and G‐box (López‐Ochoa et al., 2007), which suggested that the expression of these genes might be light dependent. Additionally, several of the TPS gene promoters contained elements for hormone‐mediated regulation of elements, such as ABREs, EREs, and MYC. This finding is consistent with previous findings concerning TPS gene promoters (Yang et al., 2023). The identification of a larger number of TPS genes was also observed in a study on volatiles performed by GC‒MS, in which many terpenoids were identified. Several biochemically studies are need to characterize the TPS genes and other genes involved in terpenoid biochemistry to provide a direct link between the TPS genes and the metabolites they produce.
The three well‐characterized genomes published here from the large genus Polyscias lay the foundation for further genomic and biochemical studies, among others. Whole‐genome sequences provide a valuable source for future studies of traits, commercial utilization of any kind, biochemical studies, and many other studies related to the evolution of plants. In this study, three genomes within a large plant family were characterized and made publicly available. Although we performed a large amount of work on the sequencing of members of the genus Polyscias, there are more plants in the order Apiales that also generate many secondary metabolites that have attracted the interest of researchers. Further genomic analysis of Apiales is needed to expand our knowledge of Polyscias and closely related species.
AUTHOR CONTRIBUTIONS
Mingzhou Bai: Data curation; formal analysis; investigation; methodology; validation; visualization; writing—original draft; writing—review and editing. Xin Yang: Data curation; formal analysis; investigation. David H. Lorence: Data curation; formal analysis; investigation. Kenneth R. Wood: Data curation; formal analysis; investigation. Natalie Iwanycki Ahlstrand: Data curation; investigation. Timothy W. Flynn: Data curation; formal analysis; investigation. Shancen Zhao: Conceptualization; funding acquisition; project administration; supervision. Nina Rønsted: Conceptualization; funding acquisition; project administration; supervision; validation; writing—review and editing. Henrik Toft Simonsen: Conceptualization; formal analysis; funding acquisition; investigation; project administration; resources; supervision; validation; writing—review and editing.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Supporting information
Figure S1: Genomic BUSCO assessment of P. cf. bisattenuata, P. lallanii and P. macgillivrayi.
Figure S2: Sequence divergence rates of four different TEs according to RepeatMasker annotation.
Figure S3: Sequence divergence rates of four different TEs via de novo annotation.
Figure S4: Statistics of gene structure prediction.
Figure S5: GO function annotation statistics.
Figure S6: Distribution of gene function annotations in five functional databases.
Figure S7: Phylogenetic tree and estimation of the divergence times of Polyscias cf. bisattenuata, Polyscias lallanii, Polyscias macgillivrayi and 13 other plant species.
Figure S8: Distribution of homologous genes in Polyscias cf. bisattenuata, Polyscias lallanii, Polyscias macgillivrayi and 13 other plant species.
Figure S9. GO functional enrichment of expanded gene families.
Figure S10: KEGG functional enrichment of expanded gene families.
Figure S11: Phylogenetic tree of the TPS‐a gene subfamily in different species.
Figure S12: Phylogenetic tree of the TPS‐b gene subfamily in different species.
Figure S13: Phylogenetic tree of the TPS‐c and TPS‐e/f gene subfamilies in different species.
Figure S14: Phylogenetic tree of the TPS‐g gene subfamily in different species.
Figure S15: Phylogenetic analysis of TPS genes from the Polyscias cf. bisattenuata, Polyscias lallanii, Polyscias macgillivrayi, Aralia elata, Panax ginseng, Panax japonicus, Panax notoginseng, Panax quinquefolius, Panax stipuleanatus and Daucus carota genomes.
Figure S16: Phylogenetic relationships, gene structure and conserved motif analyses for Aralia elata, Panax ginseng, Panax japonicus, Panax notoginseng, Panax quinquefolius and Panax stipuleanatus.
Figure S17: Synteny analysis of the TPS gene cluster.
Table S1. All Sample information.
Table S2: Statistics of BGISeq sequencing data.
Table S3: Statistics of PacBio SMRT sequencing data.
Table S4. Statistics of MGISeq transcriptome sequencing data.
Table S5. Genome completion evaluation using mapping coverage of the transcriptome.
Table S6: Statistics of P. cf. bisattenuata assemblies.
Table S7: Statistics of P. lallanii assemblies.
Table S8: Statistics of P. macgillivrayi assemblies.
Table S9: Evaluation of the genome via BUSCO single‐copy orthologs.
Table S10: Statistics of gene annotations.
Table S11: Repeat statistics of P. cf. bisattenuata, P. lallanii, and P. macgillivrayi.
Table S12: Evaluation of genes via BUSCO single‐copy orthologs.
Table S13: Comparison of gene families clustered by OrthoMCL in 16 species.
Table S14: Kernel function analysis of the Ks peak distribution from inter‐ and intragenomic comparisons.
Table S15: Statistics of gene functional annotation.
Table S16: KEGG enrichment analysis of expanded gene families in P. cf. bisattenuata, P. lallanii, and P. macgillivrayi (Q value < 0.01)
Table S17: KEGG enrichment analysis of contracted gene families in P. cf. bisattenuata, P. lallanii, and P. macgillivrayi (Q value < 0.01).
Table S18: GenBank IDs of the proteins used for phylogenetic tree construction in this study.
Table S19: The TPS gene IDs of the proteins used for phylogenetic tree construction in this study.
Table S20: Full name list of the gene abbreviations.
ACKNOWLEDGMENTS
The collection trip performed at Kauaʻi was supported by the Carlsberg Foundation (grant number CF21‐0128). The authors would like to thank all the staff at NTBG for their support throughout the project. The population sampling for P. cf. bisattenuata was supported by grant #G21AC10773 from the US Geological Survey and a Maxwell–Hanrahan Field Research Internship.
Bai, M. , Yang, X. , Lorence, D. H. , Wood, K. R. , Ahlstrand, N. I. , Flynn, T. W. , Zhao, S. , Rønsted, N. , & Simonsen, H. T. (2025). Genome sequencing of three Polyscias species reveals common features in terpene synthase gene family evolution in these species. The Plant Genome, 18, e20563. 10.1002/tpg2.20563
Assigned to Associate Editor Davoud Torkamaneh.
Contributor Information
Nina Rønsted, Email: nronsted@snm.ku.dk.
Henrik Toft Simonsen, Email: henrik.toft.simonsen@univ-st-etienne.fr.
DATA AVAILABILITY STATEMENT
The raw genome and transcriptome sequencing data for P. cf. bisattenuata, P. macgillivrayi, and P. lallanii have been deposited in the NCBI database under BioProject accession number PRJNA1079399.
REFERENCES
- Adams, J. W. A. (2016a). Polyscias waimeae . The IUCN Red List of Threatened Species 2016: e.T97821101A97821105. 10.2305/IUCN.UK.2016-2.RLTS.T97821101A97821105.en [DOI]
- Adams, J. W. A. (2016b). Polyscias racemosa . The IUCN Red List of Threatened Species 2016: e.T34055A83787166. 10.2305/IUCN.UK.2016-2.RLTS.T34055A83787166.en [DOI]
- Adams, J. W. A. (2016c). Polyscias waialealae . The IUCN Red List of Threatened Species 2016: e.T97820728A97820800. 10.2305/IUCN.UK.2016-2.RLTS.T97820728A97820800.en [DOI]
- Ashburner, M. , Ball, C A. , Blake, J. A. , Botstein, D. , Butler, H. , Cherry, J. M. , Davis, A. P. , Dolinski, K. , Dwight, S. S. , Eppig, J. T. , Harris, M. A. , Hill, D. P. , Issel‐Tarver, L. , Kasarskis, A. , Lewis, S. , Matese, J. C. , Richardson, J. E. , Ringwald, M. , Rubin, G. M. , & Sherlock, G. (2000). Gene ontology: Tool for the unification of biology. Nature Genetics, 25(1), 25–29. 10.1038/75556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmawy, N. S. , Gad, H. A. , Ashour, M. L. , El‐Ahmady, S. H. , & Singab, A. N. B. (2020). The genus Polyscias (Araliaceae): A phytochemical and biological review. Journal of Herbal Medicine, 23, Article 100377. 10.1016/j.hermed.2020.100377 [DOI] [Google Scholar]
- Aubourg, S. , Lecharny, A. , & Bohlmann, J. (2002). Genomic analysis of the terpenoid synthase (AtTPS) gene family of Arabidopsis thaliana . Molecular Genetics and Genomics, 267, 730–745. 10.1007/s00438-002-0709-y [DOI] [PubMed] [Google Scholar]
- Bailey, T. L. , Boden, M. , Buske, F. A. , Frith, M. , Grant, C. E. , Clementi, L. , Ren, J. , Li, W. W. , & Noble, W. S. (2009). MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Research, 37(Suppl_2), W202–W208. 10.1093/nar/gkp335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao, Z. , & Eddy, S. R. (2002). Automated de novo identification of repeat sequence families in sequenced genomes. Genome Research, 12(8), 1269–1276. 10.1101/gr.88502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker, M. S. , Arrigo, N. , Baniaga, A. E. , Li, Z. , & Levin, D. A. (2016). On the relative abundance of autopolyploids and allopolyploids. New Phytologist, 210(2), 391–398. 10.1111/nph.13698 [DOI] [PubMed] [Google Scholar]
- Bauer, S. , Grossmann, S. , Vingron, M. , & Robinson, P. N. (2008). Ontologizer 2.0—A multifunctional tool for GO term enrichment analysis and data exploration. Bioinformatics, 24(14), 1650–1651. 10.1093/bioinformatics/btn250 [DOI] [PubMed] [Google Scholar]
- Benson, G. (1999). Tandem repeats finder: A program to analyze DNA sequences. Nucleic Acids Research, 27(2), 573–580. 10.1093/nar/27.2.573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeckmann, B. , Bairoch, A. , Apweiler, R. , Blatter, M. C. , Estreicher, A. , Gasteiger, E. , Martin, M. J. , Michoud, K. , O'Donovan, C. , Phan, I. , Pilbout, S. , & Schneider, M. (2003). The SWISS‐PROT protein knowledgebase and its supplement TrEMBL in 2003. Nucleic Acids Research, 31(1), 365–370. 10.1093/nar/gkg095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutanaev, A. M. , Moses, T. , Zi, J. , Nelson, D. R. , Mugford, S. T. , Peters, R. J. , & Osbourn, A. (2015). Investigation of terpene diversification across multiple sequenced plant genomes. Proceedings of the National Academy of Sciences of the United States of America, 112(1), E81–E88. 10.1073/pnas.1419547112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cañestro, C. , Albalat, R. , Irimia, M. , & Garcia‐Fernàndez, J. (2013). Impact of gene gains, losses and duplication modes on the origin and diversification of vertebrates. Seminars in Cell & Developmental Biology, 24(2), 83–94. [DOI] [PubMed] [Google Scholar]
- Cárdenas, P. D. , Almeida, A. , & Bak, S. (2019). Evolution of structural diversity of triterpenoids. Frontiers in Plant Science, 10, Article 486054. 10.3389/fpls.2019.01523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castresana, J. (2000). Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Molecular Biology and Evolution, 17(4), 540–552. 10.1093/oxfordjournals.molbev.a026334 [DOI] [PubMed] [Google Scholar]
- Chaw, S.‐M. , Liu, Y.‐C. , Wu, Y.‐W. , Wang, H.‐Y. , Lin, C.‐Y. I. , Wu, C.‐S. , Ke, H.‐M. , Chang, L.‐Y. , Hsu, C.‐Y. , Yang, H.‐T. , Sudianto, E. , Hsu, M.‐H. , Wu, K.‐P. , Wang, L.‐N. , Leebens‐Mack, J. H. , & Tsai, I. J. (2019). Stout camphor tree genome fills gaps in understanding of flowering plant genome evolution. Nature Plants, 5(1), 63–73. 10.1038/s41477-018-0337-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, C. , Chen, H. , Zhang, Y. , Thomas, H. R. , Frank, M. H. , He, Y. , & Xia, R. (2020). TBtools: An integrative toolkit developed for interactive analyses of big biological data. Molecular Plant, 13(8), 1194–1202. 10.1016/j.molp.2020.06.009 [DOI] [PubMed] [Google Scholar]
- Chen, F. , Tholl, D. , Bohlmann, J. , & Pichersky, E. (2011). The family of terpene synthases in plants: A mid‐size family of genes for specialized metabolism that is highly diversified throughout the kingdom. The Plant Journal, 66(1), 212–229. 10.1111/j.1365-313x.2011.04520.x [DOI] [PubMed] [Google Scholar]
- Cheng, H. , Jarvis, E. D. , Fedrigo, O. , Koepfli, K.‐P. , Urban, L. , Gemmell, N. J. , & Li, H. (2022). Haplotype‐resolved assembly of diploid genomes without parental data. Nature Biotechnology, 40(9), 1332–1335. 10.1038/s41587-022-01261-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bie, T. , Cristianini, N. , Demuth, J P. , & Hahn, M W. (2006). CAFE: A computational tool for the study of gene family evolution. Bioinformatics, 22(10), 1269–1271. 10.1093/bioinformatics/btl097 [DOI] [PubMed] [Google Scholar]
- Do, V.‐M. , Tran, C.‐L. , Duong, T.‐H. , Phan, H.‐V.‐T. , Nguyen, H.‐H. , Nguyen, T.‐P. , & Sichaem, J. (2021). Chemical constituents of the leaves of Polyscias fruticosa . Chemistry of Natural Compounds, 57(6), 1125–1127. 10.1007/s10600-021-03566-w [DOI] [Google Scholar]
- Eaton, A. L. , Brodie, P. J. , Callmander, M. W. , Rakotondrajaona, R. , Rakotobe, E. , Rasamison, V. E. , & Kingston, D. G. (2015). Bioactive oleanane glycosides from Polyscias duplicata from the Madagascar dry forest. Natural Product Communications, 10(4), Article 1934578X1501000407. 10.1177/1934578X1501000407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellinghaus, D. , Kurtz, S. , & Willhoeft, U. (2008). LTRharvest, an efficient and flexible software for de novo detection of LTR retrotransposons. BMC Bioinformatics, 9, Article 18. 10.1186/1471-2105-9-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emms, D. M. , & Kelly, S. (2015). OrthoFinder: Solving fundamental biases in whole genome comparisons dramatically improves orthogroup inference accuracy. Genome Biology, 16, Article 157. 10.1186/s13059-015-0721-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, K. , Yan, Y.‐J. , Sun, N. , Yang, Z.‐Y. , Zhao, S.‐P. , Wu, P. , & Li, L.‐J. (2024). Exogenous methyl jasmonate treatment induced the transcriptional responses and accumulation of volatile terpenoids in Oenanthe javanica (Blume) DC. International Journal of Biological Macromolecules, 265, Article 131017. 10.1016/j.ijbiomac.2024.131017 [DOI] [PubMed] [Google Scholar]
- Finn, R. D. , Clements, J. , & Eddy, S. R. (2011). HMMER web server: Interactive sequence similarity searching. Nucleic Acids Research, 39(Suppl_2), W29–W37. 10.1093/nar/gkr367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester, N J. , Rebolleda‐Gómez, M. , Sachs, J L. , & Ashman, T.‐L. (2020). Polyploid plants obtain greater fitness benefits from a nutrient acquisition mutualism. New Phytologist, 227(3), 944–954. 10.1111/nph.16574 [DOI] [PubMed] [Google Scholar]
- French, B. R. (1986). Food plants of Papua New Guinea: A compendium . https://www.echocommunity.org/id/resources/a585517c‐260f‐47e5‐871b‐8a020f125e7e
- Guan, D. , McCarthy, S. A. , Wood, J. , Howe, K. , Wang, Y. , & Durbin, R. (2020). Identifying and removing haplotypic duplication in primary genome assemblies. Bioinformatics, 36(9), 2896–2898. 10.1093/bioinformatics/btaa025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn, M. A. , Van Kleunen, M. , & Müller‐Schärer, H. (2012). Increased phenotypic plasticity to climate may have boosted the invasion success of polyploid Centaurea stoebe . PLoS ONE, 7(11), Article e50284. 10.1371/journal.pone.0050284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada, K. , Zou, C. , Lehti‐Shiu, M. D. , Shinozaki, K. , & Shiu, S.‐H. (2008). Importance of lineage‐specific expansion of plant tandem duplicates in the adaptive response to environmental stimuli. Plant Physiology, 148(2), 993–1003. 10.1104/pp.108.122457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, J. , Verstappen, F. , Jiao, A. , Dicke, M. , Bouwmeester, H. J. , & Kappers, I. F. (2022). Terpene synthases in cucumber (Cucumis sativus) and their contribution to herbivore‐induced volatile terpenoid emission. New Phytologist, 233(2), 862–877. 10.1111/nph.17814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges, S. , Dudley, J. T. , & Kumar, S. (2006). TimeTree: A public knowledge‐base of divergence times among organisms. Bioinformatics, 22(23), 2971–2972. 10.1093/bioinformatics/btl505 [DOI] [PubMed] [Google Scholar]
- Holopainen, J. K. , Himanen, S. J. , Yuan, J. , Chen, F. , & Stewart, C. N. (2013). Ecological functions of terpenoids in changing climates. In Ramawat K. & Mérillon M. (Eds.), Natural products (pp. 2913–2940). Springer. [Google Scholar]
- Holt, C. , & Yandell, M. (2011). MAKER2: An annotation pipeline and genome‐database management tool for second‐generation genome projects. BMC Bioinformatics, 12, Article 491. 10.1186/1471-2105-12-491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huan, V. D. , Yamamura, S. , Ohtani, K. , Kasai, R. , Yamasaki, K. , Nham, N. T. , & Chau, H. M. (1998). Oleanane saponins from Polyscias fruticosa . Phytochemistry, 47(3), 451–457. 10.1016/s0031-9422(97)00618-3 [DOI] [PubMed] [Google Scholar]
- Hunter, S. , Apweiler, R. , Attwood, T. K. , Bairoch, A. , Bateman, A. , Binns, D. , Bork, P. , Das, U. , Daugherty, L. , Duquenne, L. , Finn, R. D. , Gough, J. , Haft, D. , Hulo, N. , Kahn, D. , Kelly, E. , Laugraud, A. , Letunic, I. , Lonsdale, D. , … Yeats, C. (2009). InterPro: The integrative protein signature database. Nucleic Acids Research, 37(Suppl_1), D211–D215. 10.1093/nar/gkn785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husband, B. C. , Baldwin, S. J. , & Suda, J. (2013). The incidence of polyploidy in natural plant populations: Major patterns and evolutionary processes. In Greilhuber J., Dolezel J., & Wendel J. (Eds.), Plant genome diversity: Volume 2: Physical structure, behaviour and evolution of plant genomes (pp. 255–276). Springer. 10.1007/978-3-7091-1160-4_16 [DOI] [Google Scholar]
- International Union for Conservation of Nature (IUCN) . (2024). Polyscias . The IUCN Red List of Threatened Species, Version 2023‐1. https://www.iucnredlist.org
- Iorizzo, M. , Ellison, S. , Senalik, D. , Zeng, P. , Satapoomin, P. , Huang, J. , Bowman, M. , Iovene, M. , Sanseverino, W. , Cavagnaro, P. , Yildiz, M. , Macko‐Podgórni, A. , Moranska, E. , Grzebelus, E. , Grzebelus, D. , Ashrafi, H. , Zheng, Z. , Cheng, S. , Spooner, D. , … Simon, P. (2016). A high‐quality carrot genome assembly provides new insights into carotenoid accumulation and asterid genome evolution. Nature Genetics, 48(6), 657–666. 10.1038/ng.3565 [DOI] [PubMed] [Google Scholar]
- Jia, Q. , Brown, R. , Köllner, T. G. , Fu, J. , Chen, X. , Wong, G. K.‐S. , Gershenzon, J. , Peters, R. J. , & Chen, F. (2022). Origin and early evolution of the plant terpene synthase family. Proceedings of the National Academy of Sciences of the United States of America, 119(15), Article e2100361119. 10.1073/pnas.2100361119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimbo, T. (2022). Polyscias macgillivrayi . The IUCN Red List of Threatened Species 2022: e.T198374493A202836654. 10.2305/IUCN.UK.2022-1.RLTS.T198374493A202836654.en [DOI]
- Johnson, A. D. , Handsaker, R. E. , Pulit, S. L. , Nizzari, M. M. , O'Donnell, C. J. , & De Bakker, P. I. (2008). SNAP: A web‐based tool for identification and annotation of proxy SNPs using HapMap. Bioinformatics, 24(24), 2938–2939. 10.1093/bioinformatics/btn564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurka, J. , Kapitonov, V. V. , Pavlicek, A. , Klonowski, P. , Kohany, O. , & Walichiewicz, J. (2005). Repbase Update, a database of eukaryotic repetitive elements. Cytogenetic and Genome Research, 110(1‐4), 462–467. 10.1159/000084979 [DOI] [PubMed] [Google Scholar]
- Kanehisa, M. , & Goto, S. (2000). KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Research, 28(1), 27–30. 10.1093/nar/28.1.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, J. S. , Giang, V. N. L. , Park, H. S. , Park, Y. S. , Cho, W. , Nguyen, V. B. , Shim, H. , Waminal, N. E. , Park, J. Y. , Kim, H. H. , & Yang, T. J. (2023). Evolution of the Araliaceae family involved rapid diversification of the Asian Palmate group and Hydrocotyle specific mutational pressure. Scientific Reports, 13, 22325. 10.1038/s41598-023-49830-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunanithi, P. S. , & Zerbe, P. (2019). Terpene synthases as metabolic gatekeepers in the evolution of plant terpenoid chemical diversity. Frontiers in Plant Science, 10, Article 1166. 10.3389/fpls.2019.01166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh, K. , Misawa, K. , Kuma, K.‐i. , & Miyata, T. (2002). MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research, 30(14), 3059–3066. 10.1093/nar/gkf436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kautsar, S A. , Suarez Duran, H G. , Blin, K. , Osbourn, A. , & Medema, M H. (2017). PlantiSMASH: Automated identification, annotation and expression analysis of plant biosynthetic gene clusters. Nucleic Acids Research, 45(W1), W55–W63. 10.1093/nar/gkx305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilwagen, J. , Hartung, F. , Paulini, M. , Twardziok, S. O. , & Grau, J. (2018). Combining RNA‐seq data and homology‐based gene prediction for plants, animals and fungi. BMC Bioinformatics, 19, Article 189. 10.1186/s12859-018-2203-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, D. , Langmead, B. , & Salzberg, S. L. (2015). HISAT: A fast spliced aligner with low memory requirements. Nature Methods, 12(4), 357–360. 10.1038/nmeth.3317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonin, E. V. , Fedorova, N. D. , Jackson, J. D. , Jacobs, A. R. , Krylov, D. M. , Makarova, K. S. , Mazumder, R. , Mekhedov, S. L. , Nikolskaya, A. N. , Rao, B. S. , Rogozin, I. B. , Smirnov, S. , Sorokin, A. V. , Sverdlov, A. V. , Vasudevan, S. , Wolf, Y. I. , Yin, J. J. , & Natale, D. A. (2004). A comprehensive evolutionary classification of proteins encoded in complete eukaryotic genomes. Genome Biology, 5, Article R7. 10.1186/gb-2004-5-2-r7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurup, R. , Sadasivan, A. N. , Kalavathy, U. , & Baby, S. (2020). Chemical profile and anticancer activity of Polyscias guilfoylei leaf essential oil. The Natural Products Journal, 10(4), 372–383. 10.2174/2210315509666190624103355 [DOI] [Google Scholar]
- Leitch, A. , & Leitch, I. (2008). Genomic plasticity and the diversity of polyploid plants. Science, 320(5875), 481–483. 10.1126/science.1153585 [DOI] [PubMed] [Google Scholar]
- Lescot, M. (2002). PlantCARE, a database of plant cis‐acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Research, 30(1), 325–327. 10.1093/nar/30.1.325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letunic, I. , & Bork, P. (2021). Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Research, 49(W1), W293–W296. 10.1093/nar/gkab301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H. (2013). Aligning sequence reads, clone sequences and assembly contigs with BWA‐MEM. arXiv. 10.48550/arXiv.1303.3997 [DOI]
- Li, H. , Handsaker, B. , Wysoker, A. , Fennell, T. , Ruan, J. , Homer, N. , Marth, G. , Abecasis, G. , Durbin, R. , & 1000 Genome Project Data Processing Subgroup . (2009). The sequence alignment/map format and SAMtools. Bioinformatics, 25(16), 2078–2079. 10.1093/bioinformatics/btp352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, M.‐R. , Ding, N. , Lu, T. , Zhao, J. , Wang, Z.‐H. , Jiang, P. , Liu, S.‐T. , Wang, X.‐F. , Liu, B. , & Li, L.‐F. (2021). Evolutionary contribution of duplicated genes to genome evolution in the ginseng species complex. Genome Biology and Evolution, 13(5), Article evab051. 10.1093/gbe/evab051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, M.‐Y. , Feng, K. , Hou, X.‐L. , Jiang, Q. , Xu, Z.‐S. , Wang, G.‐L. , Liu, J.‐X. , Wang, F. , & Xiong, A.‐S. (2020). The genome sequence of celery (Apium graveolens L.), an important leaf vegetable crop rich in apigenin in the Apiaceae family. Horticulture Research, 7, Article 9. 10.1038/s41438-019-0235-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López‐Ochoa, L. , Acevedo‐Hernández, G. , Martínez‐Hernández, A. , Argüello‐Astorga, G. , & Herrera‐Estrella, L. (2007). Structural relationships between diverse cis‐acting elements are critical for the functional properties of a rbcS minimal light regulatory unit. Journal of Experimental Botany, 58(15‐16), 4397–4406. 10.1093/jxb/erm307 [DOI] [PubMed] [Google Scholar]
- Lowry, P. P. , & Plunkett, G. M. (2010). Recircumscription of Polyscias (Araliaceae) to include six related genera, with a new infrageneric classification and a synopsis of species. Plant Diversity and Evolution, 128(1‐2), 55–84. 10.1127/1869-6155/2010/0128-0003 [DOI] [Google Scholar]
- Ma, L. , Liu, K.‐W. , Li, Z. , Hsiao, Y.‐Y. , Qi, Y. , Fu, T. , Tang, G.‐D. , Zhang, D. , Sun, W.‐H. , Liu, D.‐K. , Li, Y. , Chen, G.‐Z. , Liu, X.‐D. , Liao, X.‐Y. , Jiang, Y.‐T. , Yu, X. , Hao, Y. , Huang, J. , Zhao, X.‐W. , … Liu, Z.‐J. (2023). Diploid and tetraploid genomes of Acorus and the evolution of monocots. Nature Communications, 14(1), Article 3661. 10.1038/s41467-023-38829-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marçais, G. , & Kingsford, C. (2011). A fast, lock‐free approach for efficient parallel counting of occurrences of k‐mers. Bioinformatics, 27(6), 764–770. 10.1093/bioinformatics/btr011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, D. , Tholl, D. , Gershenzon, J. , & Bohlmann, J. r. (2002). Methyl jasmonate induces traumatic resin ducts, terpenoid resin biosynthesis, and terpenoid accumulation in developing xylem of Norway spruce stems. Plant Physiology, 129(3), 1003–1018. 10.1104/pp.011001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, D. M. , Aubourg, S. , Schouwey, M. B. , Daviet, L. , Schalk, M. , Toub, O. , Lund, S. T. , & Bohlmann, J. (2010). Functional annotation, genome organization and phylogeny of the grapevine (Vitis vinifera) terpene synthase gene family based on genome assembly, FLcDNA cloning, and enzyme assays. BMC Plant Biology, 10, Article 226. 10.1186/1471-2229-10-226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez‐Swatson, K. , Quiñonero‐López, C. , Ernst, M. , Rønsted, N. , Barnes, C. J. , & Simonsen, H. T. (2020). Thapsigargins and induced chemical defence in Thapsia garganica . Chemoecology, 30(5), 255–267. 10.1007/s00049-020-00315-3 [DOI] [Google Scholar]
- Minh, B. Q. , Schmidt, H. A. , Chernomor, O. , Schrempf, D. , Woodhams, M. D. , Von Haeseler, A. , & Lanfear, R. (2020). IQ‐TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Molecular Biology and Evolution, 37(5), 1530–1534. 10.1093/molbev/msaa015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misener, S. , & Krawetz, S. A. (Eds.). (2000). Bioinformatics methods and protocols (Vol. 132). Springer. 10.1385/1592591922 [DOI] [Google Scholar]
- Mitaine‐Offer, A. , Tapondjou, L. , Lontsi, D. , Sondengam, B. , Choudhary, M. , Atta‐ur‐Rahman, M. , & Lacaille‐Dubois, M. (2004). Constituents isolated from Polyscias fulva . Biochemical Systematics and Ecology, 32(6), 607–610. 10.1016/j.bse.2003.10.008 [DOI] [Google Scholar]
- Nagegowda, D. A. , & Gupta, P. (2020). Advances in biosynthesis, regulation, and metabolic engineering of plant specialized terpenoids. Plant Science, 294, Article 110457. 10.1016/j.plantsci.2020.110457 [DOI] [PubMed] [Google Scholar]
- Naghiloo, S. , & Vamosi, J. (2023). Are polyploid species less vulnerable to climate change? A case study in North American Crataegus . American Journal of Climate Change, 12(3), 359–375. 10.4236/ajcc.2023.123017 [DOI] [Google Scholar]
- Neumann, P. , Novák, P. , Hoštáková, N. , & Macas, J. (2019). Systematic survey of plant LTR‐retrotransposons elucidates phylogenetic relationships of their polyprotein domains and provides a reference for element classification. Mobile DNA, 10, Article 1. 10.1186/s13100-018-0144-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninkuu, V. , Zhang, L. , Yan, J. , Fu, Z. , Yang, T. , & Zeng, H. (2021). Biochemistry of terpenes and recent advances in plant protection. International Journal of Molecular Sciences, 22(11), Article 5710. 10.3390/ijms22115710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou, S. , & Jiang, N. (2018). LTR_retriever: A highly accurate and sensitive program for identification of long terminal repeat retrotransposons. Plant Physiology, 176(2), 1410–1422. 10.1104/pp.17.01310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertea, M. , Pertea, G. M. , Antonescu, C. M. , Chang, T.‐C. , Mendell, J. T. , & Salzberg, S. L. (2015). StringTie enables improved reconstruction of a transcriptome from RNA‐seq reads. Nature Biotechnology, 33(3), 290–295. 10.1038/nbt.3122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichersky, E. , & Raguso, R. A. (2018). Why do plants produce so many terpenoid compounds? New Phytologist, 220(3), 692–702. 10.1111/nph.14178 [DOI] [PubMed] [Google Scholar]
- Plants of the World Online . (n.d.). Polyscias macgillivrayi (Benth.) Harms . https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:847355‐1
- Price, A. L. , Jones, N. C. , & Pevzner, P. A. (2005). De novo identification of repeat families in large genomes. Bioinformatics, 21(Suppl_1), i351–i358. 10.1093/bioinformatics/bti1018 [DOI] [PubMed] [Google Scholar]
- Quattrocchi, U. (2012). CRC world dictionary of medicinal and poisonous plants: Common names, scientific names, eponyms, synonyms, and etymology (5 volume set). CRC Press. 10.1201/b16504 [DOI] [Google Scholar]
- Ranallo‐Benavidez, T. R. , Jaron, K. S. , & Schatz, M. C. (2020). GenomeScope 2.0 and Smudgeplot for reference‐free profiling of polyploid genomes. Nature Communications, 11(1), Article 1432. 10.1038/s41467-020-14998-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rønsted, N. , Walsh, S K. , Clark, M. , Edmonds, M. , Flynn, T. , Heintzman, S. , Loomis, A. , Lorence, D. , Nagendra, U. , Nyberg, B. , Opgenorth, M. , Weisenberger, L. , Williams, A. , Wolkis, D. , Wood, K R. , & Keir, M. (2022). Extinction risk of the endemic vascular flora of Kauai, Hawaii, based on IUCN assessments. Conservation Biology: The Journal of the Society for Conservation Biology, 36(4), Article e13896. 10.1111/cobi.13896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson, & Michael, J. (2003). R8s: Inferring absolute rates of molecular evolution and divergence times in the absence of a molecular clock. Bioinformatics, 19(2), 301–302. 10.1093/bioinformatics/19.2.301 [DOI] [PubMed] [Google Scholar]
- Seppey, M. , Manni, M. , & Zdobnov, E. M. (2019). BUSCO: Assessing genome assembly and annotation completeness. In Kollmar M. (Ed.), Gene prediction: Methods and protocols (pp. 227–245). Springer. 10.1007/978-1-4939-9173-0_14 [DOI] [PubMed] [Google Scholar]
- Singh, R. K. (2024). Polyscias lallanii R. Kr. Singh & Sanjeet Kumar, a replacement name for P. racemosa (CN Forbes) Lowry & G. M. Plunkett (Araliaceae). Journal of Biodiversity & Conservation, 8(2), 17–22. [Google Scholar]
- Smit, S. J. , & Lichman, B. R. (2022). Plant biosynthetic gene clusters in the context of metabolic evolution. Natural Product Reports, 39(7), 1465–1482. 10.1039/D2NP00005A [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltis, P. S. , & Soltis, D. E. (2016). Ancient WGD events as drivers of key innovations in angiosperms. Current Opinion in Plant Biology, 30, 159–165. 10.1016/j.pbi.2016.03.015 [DOI] [PubMed] [Google Scholar]
- Song, X. , Wang, J. , Li, N. , Yu, J. , Meng, F. , Wei, C. , Liu, C. , Chen, W. , Nie, F. , Zhang, Z. , Gong, K. , Li, X. , Hu, J. , Yang, Q. , Li, Y. , Li, C. , Feng, S. , Guo, H. , Yuan, J. , … Wang, X. (2020). Deciphering the high‐quality genome sequence of coriander that causes controversial feelings. Plant Biotechnology Journal, 18(6), 1444–1456. 10.1111/pbi.13310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanke, M. , Keller, O. , Gunduz, I. , Hayes, A. , Waack, S. , & Morgenstern, B. (2006). AUGUSTUS: Ab initio prediction of alternative transcripts. Nucleic Acids Research, 34(Suppl_2), W435–W439. 10.1093/nar/gkl200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto, S. , Yamano, Y. , Khalil, H. E. , Otsuka, H. , Kamel, M. S. , & Matsunami, K. (2017). Chemical structures of constituents from the leaves of Polyscias balfouriana . Journal of Natural Medicines, 71, 558–563. 10.1007/s11418-017-1081-x [DOI] [PubMed] [Google Scholar]
- Supple, M. A. , & Shapiro, B. (2018). Conservation of biodiversity in the genomics era. Genome Biology, 19, Article 131. 10.1186/s13059-018-1520-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suyama, M. , Torrents, D. , & Bork, P. (2006). PAL2NAL: Robust conversion of protein sequence alignments into the corresponding codon alignments. Nucleic Acids Research, 34(Suppl_2), W609–W612. 10.1093/nar/gkl315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai, Y. , Ling, C. , Wang, C. , Wang, H. , Su, L. , Yang, L. , Jiang, W. , Yu, X. , Zheng, L. , Feng, Z. , Liu, C. , & Yuan, Y. (2020). Analysis of terpenoid biosynthesis pathways in German chamomile (Matricaria recutita) and Roman chamomile (Chamaemelum nobile) based on co‐expression networks. Genomics, 112(2), 1055–1064. 10.1016/j.ygeno.2019.10.023 [DOI] [PubMed] [Google Scholar]
- Tang, H. , Bowers, J. E. , Wang, X. , Ming, R. , Alam, M. , & Paterson, A. H. (2008). Synteny and collinearity in plant genomes. Science, 320(5875), 486–488. 10.1126/science.1153917 [DOI] [PubMed] [Google Scholar]
- Tangalin, N. (2015). Polyscias bisattenuata . The IUCN Red List of Threatened Species 2015: e.T80228720A80228754. 10.2305/IUCN.UK.2015-4.RLTS.T80228720A80228754.en [DOI]
- Thibaud‐Nissen, F. , Ouyang, S. , & Buell, C. R. (2009). Identification and characterization of pseudogenes in the rice gene complement. BMC Genomics, 10, Article 317. 10.1186/1471-2164-10-317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Fish and Wildlife Service . (2020). 5‐year status review summary and evaluation of Polyscias bisattenuata ('ohe'ohe). U.S. Fish and Wildlife Service, Federal Register, 85(48), 14240–14243. [Google Scholar]
- Wagner, W. L. , Khan, N. R. , & Lorence, D. H. (2023). Flora of the Hawaiian Islands . https://naturalhistory2.si.edu/botany/hawaiianflora/
- Wang, X. , Gao, Y. , Wu, X. , Wen, X. , Li, D. , Zhou, H. , Li, Z. , Liu, B. , Wei, J. , Chen, F. , Chen, F. , Zhang, C. , Zhang, L. , & Xia, Y. (2021). High‐quality evergreen azalea genome reveals tandem duplication‐facilitated low‐altitude adaptability and floral scent evolution. Plant Biotechnology Journal, 19(12), 2544–2560. 10.1111/pbi.13680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Tang, H. , Debarry, J. D. , Tan, X. , Li, J. , Wang, X. , Lee, T.‐H. , Jin, H. , Marler, B. , Guo, H. , Kissinger, J. C. , & Paterson, A. H. (2012). MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Research, 40(7), Article e49. 10.1093/nar/gkr1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Zhang, H. , Ri, H. C. , An, Z. , Wang, X. , Zhou, J.‐N. , Zheng, D. , Wu, H. , Wang, P. , Yang, J. , Liu, D.‐K. , Zhang, D. , Tsai, W.‐C. , Xue, Z. , Xu, Z. , Zhang, P. , Liu, Z.‐J. , Shen, H. , & Li, Y. (2022). Deletion and tandem duplications of biosynthetic genes drive the diversity of triterpenoids in Aralia elata . Nature Communications, 13(1), Article 2224. 10.1038/s41467-022-29908-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z.‐H. , Wang, X.‐F. , Lu, T. , Li, M.‐R. , Jiang, P. , Zhao, J. , Liu, S.‐T. , Fu, X.‐Q. , Wendel, J F. , Van De Peer, Y. , Liu, B. , & Li, L.‐F. (2022). Reshuffling of the ancestral core‐eudicot genome shaped chromatin topology and epigenetic modification in Panax . Nature Communications, 13(1), Article 1902. 10.1038/s41467-022-29561-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, D. C. , McGarvey, D. J. , Katahira, E. J. , & Croteau, R. (1998). Truncation of limonene synthase preprotein provides a fully active ‘pseudomature’ form of this monoterpene cyclase and reveals the function of the amino‐terminal arginine pair. Biochemistry, 37(35), 12213–12220. 10.1021/bi980854k [DOI] [PubMed] [Google Scholar]
- Xiong, H. , Wang, D. , Shao, C. , Yang, X. , Yang, J. , Ma, T. , Davis, C. C. , Liu, L. , & Xi, Z. (2022). Species tree estimation and the impact of gene loss following whole‐genome duplication. Systematic Biology, 71(6), 1348–1361. 10.1093/sysbio/syac040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, J. , Kong, L. , Ren, W. , Wang, Z. , Tang, L. , Wu, W. , Liu, X. , Ma, W. , & Zhang, S. (2024). Identification and expression analysis of TPS family gene in Cannabis sativa L. Heliyon, 10(6), Article e27817. 10.1016/j.heliyon.2024.e27817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Z. , & Wang, H. (2007). LTR_FINDER: An efficient tool for the prediction of full‐length LTR retrotransposons. Nucleic Acids Research, 35 (Suppl_2), W265–W268. 10.1093/nar/gkm286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada, T. , Sriprasertsak, P. , Kato, H. , Hashimoto, T. , & Shimizu, H. (1994). Functional analysis of the promoters of phenylalanine ammonia‐lyase genes in pea. Plant and Cell Physiology, 35(6), 917–926. 10.1093/oxfordjournals.pcp.a078677 [DOI] [PubMed] [Google Scholar]
- Yan, X.‐M. , Zhou, S.‐S. , Liu, H. , Zhao, S.‐W. , Tian, X.‐C. , Shi, T.‐L. , Bao, Y.‐T. , Li, Z.‐C. , Jia, K.‐H. , Nie, S. , Guo, J.‐F. , Kong, L. , Porth, I. M. , & Mao, J.‐F. (2023). Unraveling the evolutionary dynamics of the TPS gene family in land plants. Frontiers in Plant Science, 14, Article 1273648. 10.3389/fpls.2023.1273648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Z. , Chen, S. , Wang, S. , Hu, Y. , Zhang, G. , Dong, Y. , Yang, S. , Miao, J. , Chen, W. , & Sheng, J. (2021). Chromosomal‐scale genome assembly of Eleutherococcus senticosus provides insights into chromosome evolution in Araliaceae. Molecular Ecology Resources, 21(7), 2204–2220. 10.1111/1755-0998.13403 [DOI] [PubMed] [Google Scholar]
- Yang, Z. , Zhan, T. , Xie, C. , Huang, S. , & Zheng, X. (2023). Genome‐wide analyzation and functional characterization on the TPS family provide insight into the biosynthesis of mono‐terpenes in the camphor tree. Plant Physiology and Biochemistry, 196, 55–64. 10.1016/j.plaphy.2023.01.039 [DOI] [PubMed] [Google Scholar]
- Yi, T. , Lowry, P. P. , Plunkett, G. M. , & Wen, J. (2004). Chromosomal evolution in Araliaceae and close relatives. Taxon, 53(4), 987–1005. 10.2307/4135565 [DOI] [Google Scholar]
- Yin, D. , Ji, C. , Song, Q. , Zhang, W. , Zhang, X. , Zhao, K. , Chen, C Y. , Wang, C. , He, G. , Liang, Z. , Ma, X. , Li, Z. , Tang, Y. , Wang, Y. , Li, K. , Ning, L. , Zhang, H. , Zhao, K. , Li, X. , … Chen, Z. J (2020). Comparison of Arachis monticola with diploid and cultivated tetraploid genomes reveals asymmetric subgenome evolution and improvement of peanut. Advanced Science, 7(4), Article 1901672. 10.1002/advs.201901672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, H. , Cao, G. , Hou, X. , Huang, M. , Du, P. , Tan, T. , Zhang, Y. , Zhou, H. , Liu, X. , Liu, L. , Jiangfang, Y. , Li, Y. , Liu, Z. , Fang, C. , Zhao, L. , Fernie, A R. , & Luo, J. (2022). Development of a widely targeted volatilomics method for profiling volatilomes in plants. Molecular Plant, 15(1), 189–202. 10.1016/j.molp.2021.09.003 [DOI] [PubMed] [Google Scholar]
- Zapata, F. , & Fine, P. V. (2013). Diversification of the monoterpene synthase gene family (TPSb) in Protium, a highly diverse genus of tropical trees. Molecular Phylogenetics and Evolution, 68(3), 432–442. 10.1016/j.ympev.2013.04.024 [DOI] [PubMed] [Google Scholar]
- Zhou, F. , Sun, T.‐H. , Zhao, L. , Pan, X.‐W. , & Lu, S. (2015). The bZIP transcription factor HY5 interacts with the promoter of the monoterpene synthase gene QH6 in modulating its rhythmic expression. Frontiers in Plant Science, 6, Article 137218. 10.3389/fpls.2015.00304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziheng, Y. (2007). PAML 4: Phylogenetic analysis by maximum likelihood. Molecular Biology and Evolution, 24(8), 1586–1591. 10.1093/molbev/msm088 [DOI] [PubMed] [Google Scholar]
- Zwaenepoel, A. , & Van de Peer, Y. (2018). wgd—Simple command line tools for the analysis of ancient whole‐genome duplications. Bioinformatics, 35(12), 2153–2155. 10.1093/bioinformatics/bty915 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Genomic BUSCO assessment of P. cf. bisattenuata, P. lallanii and P. macgillivrayi.
Figure S2: Sequence divergence rates of four different TEs according to RepeatMasker annotation.
Figure S3: Sequence divergence rates of four different TEs via de novo annotation.
Figure S4: Statistics of gene structure prediction.
Figure S5: GO function annotation statistics.
Figure S6: Distribution of gene function annotations in five functional databases.
Figure S7: Phylogenetic tree and estimation of the divergence times of Polyscias cf. bisattenuata, Polyscias lallanii, Polyscias macgillivrayi and 13 other plant species.
Figure S8: Distribution of homologous genes in Polyscias cf. bisattenuata, Polyscias lallanii, Polyscias macgillivrayi and 13 other plant species.
Figure S9. GO functional enrichment of expanded gene families.
Figure S10: KEGG functional enrichment of expanded gene families.
Figure S11: Phylogenetic tree of the TPS‐a gene subfamily in different species.
Figure S12: Phylogenetic tree of the TPS‐b gene subfamily in different species.
Figure S13: Phylogenetic tree of the TPS‐c and TPS‐e/f gene subfamilies in different species.
Figure S14: Phylogenetic tree of the TPS‐g gene subfamily in different species.
Figure S15: Phylogenetic analysis of TPS genes from the Polyscias cf. bisattenuata, Polyscias lallanii, Polyscias macgillivrayi, Aralia elata, Panax ginseng, Panax japonicus, Panax notoginseng, Panax quinquefolius, Panax stipuleanatus and Daucus carota genomes.
Figure S16: Phylogenetic relationships, gene structure and conserved motif analyses for Aralia elata, Panax ginseng, Panax japonicus, Panax notoginseng, Panax quinquefolius and Panax stipuleanatus.
Figure S17: Synteny analysis of the TPS gene cluster.
Table S1. All Sample information.
Table S2: Statistics of BGISeq sequencing data.
Table S3: Statistics of PacBio SMRT sequencing data.
Table S4. Statistics of MGISeq transcriptome sequencing data.
Table S5. Genome completion evaluation using mapping coverage of the transcriptome.
Table S6: Statistics of P. cf. bisattenuata assemblies.
Table S7: Statistics of P. lallanii assemblies.
Table S8: Statistics of P. macgillivrayi assemblies.
Table S9: Evaluation of the genome via BUSCO single‐copy orthologs.
Table S10: Statistics of gene annotations.
Table S11: Repeat statistics of P. cf. bisattenuata, P. lallanii, and P. macgillivrayi.
Table S12: Evaluation of genes via BUSCO single‐copy orthologs.
Table S13: Comparison of gene families clustered by OrthoMCL in 16 species.
Table S14: Kernel function analysis of the Ks peak distribution from inter‐ and intragenomic comparisons.
Table S15: Statistics of gene functional annotation.
Table S16: KEGG enrichment analysis of expanded gene families in P. cf. bisattenuata, P. lallanii, and P. macgillivrayi (Q value < 0.01)
Table S17: KEGG enrichment analysis of contracted gene families in P. cf. bisattenuata, P. lallanii, and P. macgillivrayi (Q value < 0.01).
Table S18: GenBank IDs of the proteins used for phylogenetic tree construction in this study.
Table S19: The TPS gene IDs of the proteins used for phylogenetic tree construction in this study.
Table S20: Full name list of the gene abbreviations.
Data Availability Statement
The raw genome and transcriptome sequencing data for P. cf. bisattenuata, P. macgillivrayi, and P. lallanii have been deposited in the NCBI database under BioProject accession number PRJNA1079399.


