Abstract
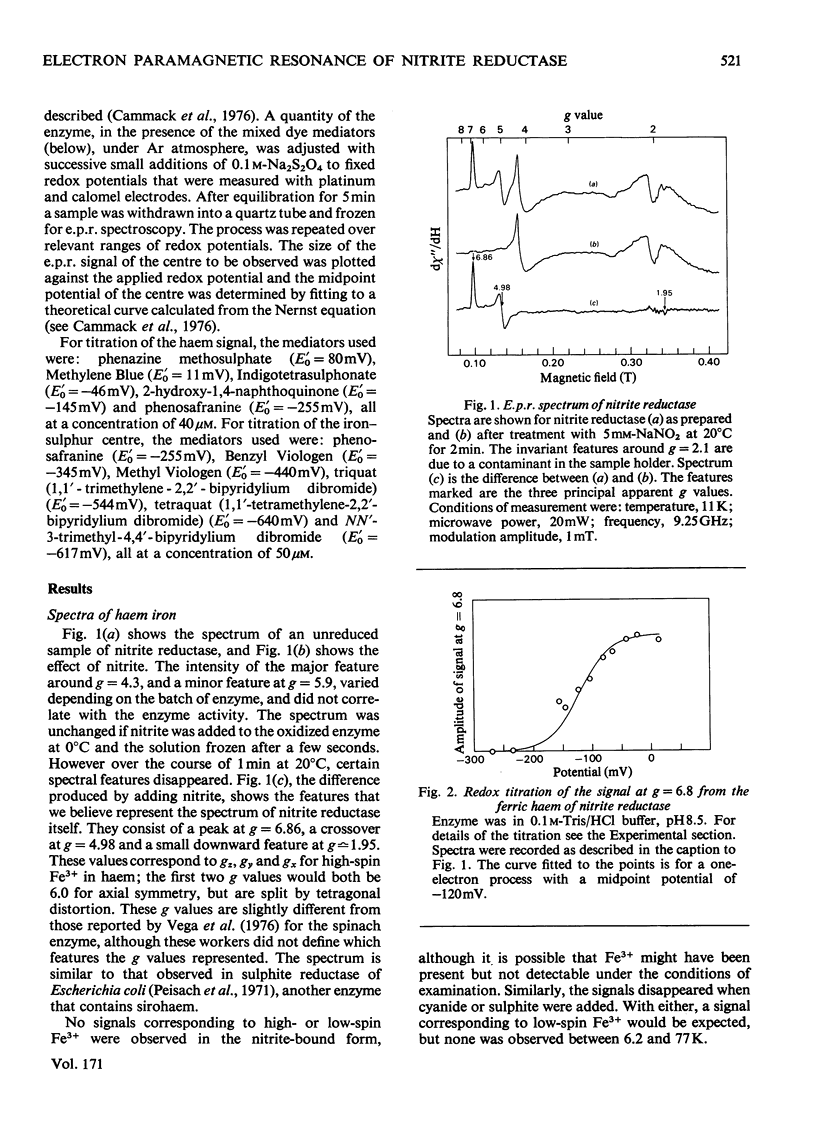
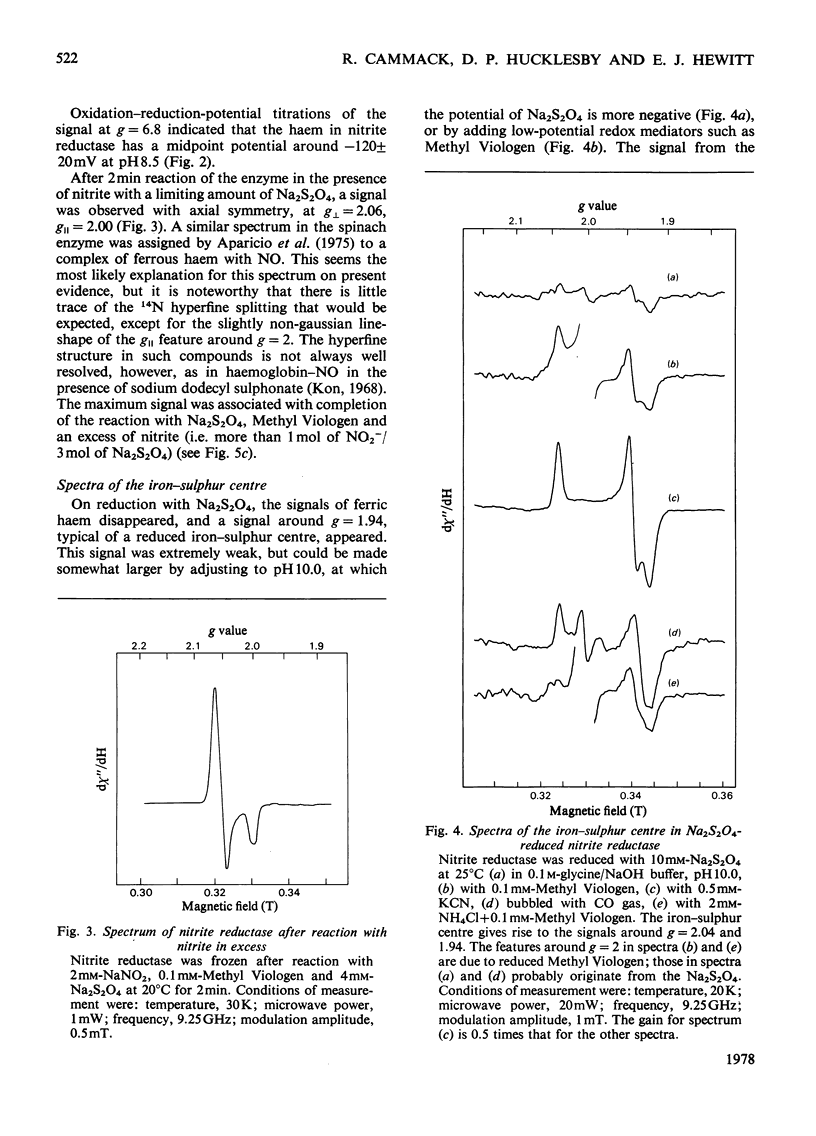
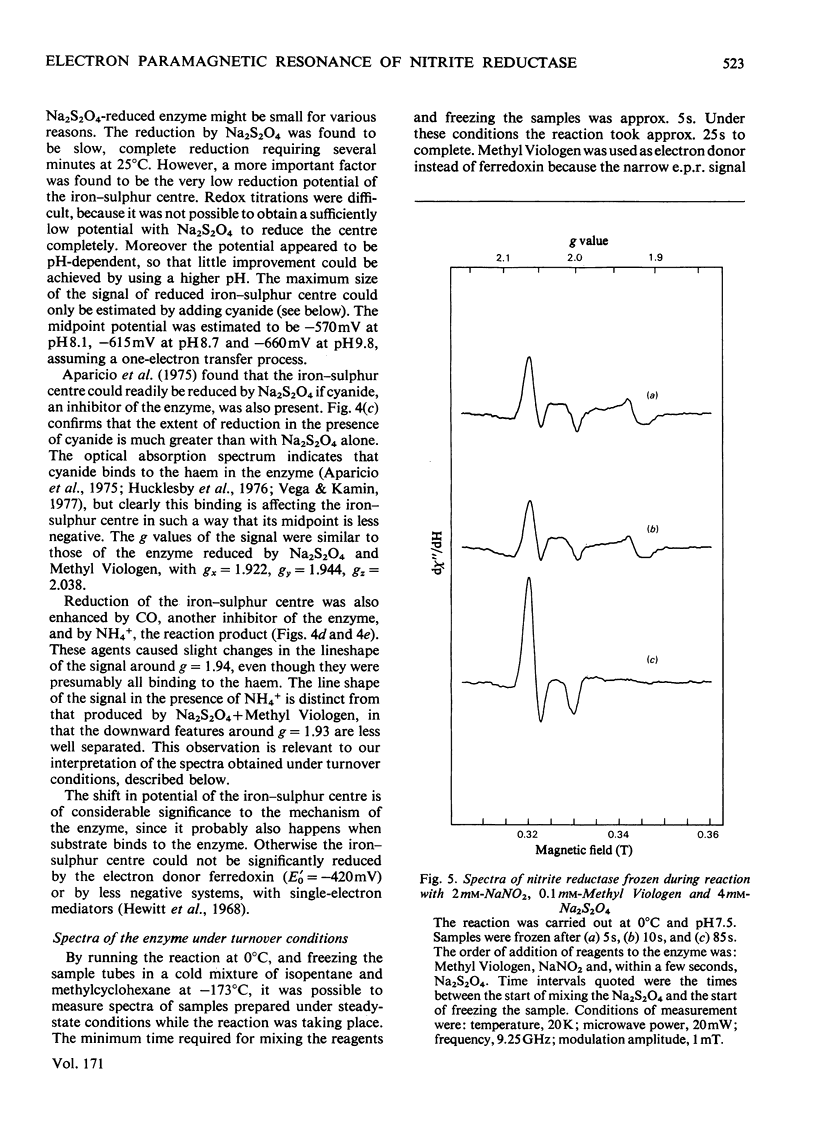
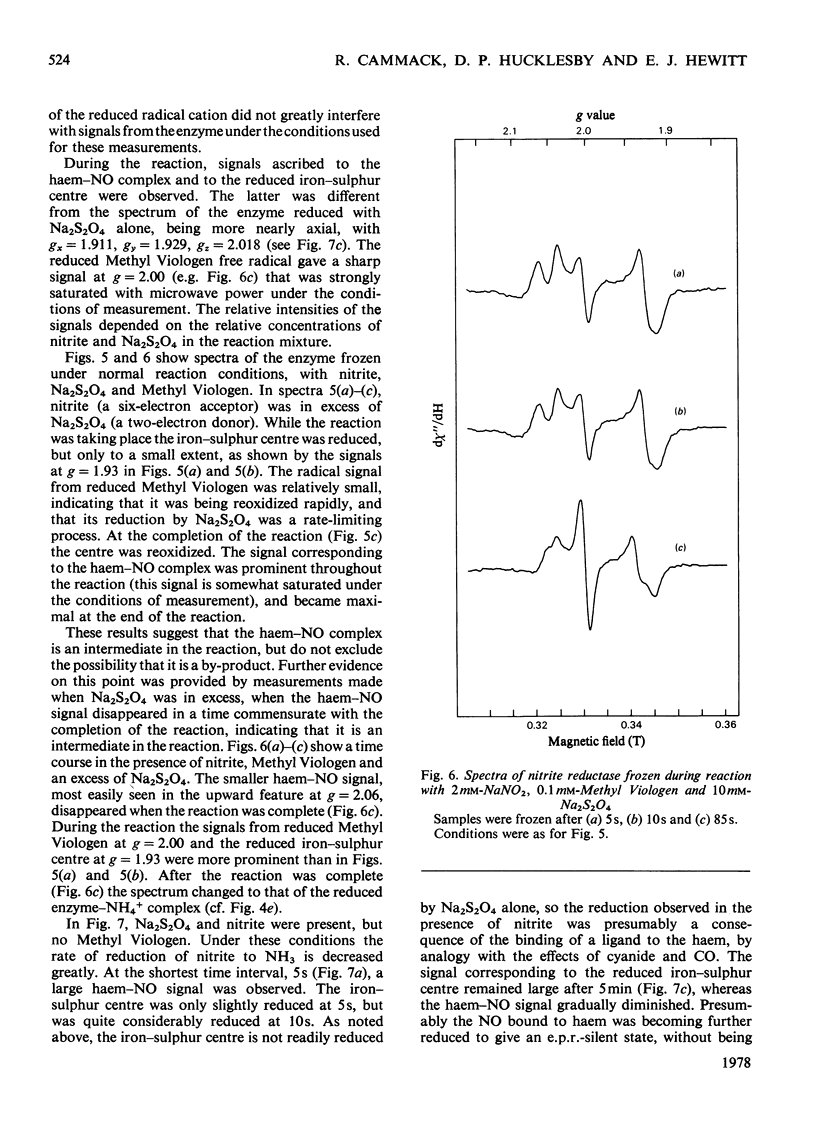
Low-temperature e.p.r. spectra are presented of nitrite reductase purified from leaves of vegetable marrow (Cucurbita pepo). The oxidized enzyme showed a spectrum at g=6.86, 4.98 and 1.95 corresponding to high-spin Fe3+ in sirohaem, which disappeared slowly on treatment with nitrite. The midpoint potential of the sirohaem was estimated to be −120mV. On reduction with Na2S2O4 or Na2S2O4+Methyl Viologen a spectrum at g=2.038, 1.944 and 1.922 was observed, due to a reduced iron–sulphur centre. The midpoint potential of this centre was very low, about −570mV at pH8.1, decreasing with increasing pH. On addition of cyanide, which binds to haem, and Na2S2O4, the iron–sulphur centre became further reduced. We think that this is due to an increased midpoint potential of the iron–sulphur centre. Other ligands to haem, such as CO and the reaction product NH3, had similar but less pronounced effects, and also changed the lineshape of the iron–sulphur signal. Samples were prepared of the enzyme frozen during the reaction with nitrite, Methyl Viologen and Na2S2O4 in various proportions. Signals were interpreted as due to the reduced iron–sulphur centre (with slightly different g values), a haem–NO complex and reduced Methyl Viologen. In the presence of an excess of nitrite, the haem–NO spectrum was more intense, whereas in the presence of an excess of Na2S2O4 it was weaker, and disappeared at the end of the reaction. A reaction sequence is proposed for the enzyme, in which the haem–NO complex is an intermediate, followed by other e.p.r.-silent states, leading to the production of NH4+.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aparicio P. J., Knaff D. B., Malkin R. The role of an iron-sulfur center and siroheme in spinach nitrite reductase. Arch Biochem Biophys. 1975 Jul;169(1):102–107. doi: 10.1016/0003-9861(75)90321-5. [DOI] [PubMed] [Google Scholar]
- Cammack R., Barber M. J., Bray R. C. Oxidation-reduction potentials of molybdenum, flavin and iron-sulphur centres in milk xanthine oxidase. Biochem J. 1976 Aug 1;157(2):469–478. doi: 10.1042/bj1570469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kon H. Paramagnetic resonance study of Nitric Oxide hemoglobin. J Biol Chem. 1968 Aug 25;243(16):4350–4357. [PubMed] [Google Scholar]
- Lafferty M. A., Garrett R. H. Purification and properties of the Neurospora crassa assimilatory nitrite reductase. J Biol Chem. 1974 Dec 10;249(23):7555–7567. [PubMed] [Google Scholar]
- Murphy M. J., Siegel L. M., Tove S. R., Kamin H. Siroheme: a new prosthetic group participating in six-electron reduction reactions catalyzed by both sulfite and nitrite reductases. Proc Natl Acad Sci U S A. 1974 Mar;71(3):612–616. doi: 10.1073/pnas.71.3.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul P., Kumta U. S. Reduced binding of nitric oxide in irradiated horse heart myoglobin. J Agric Food Chem. 1975 Jan-Feb;23(1):37–40. doi: 10.1021/jf60197a002. [DOI] [PubMed] [Google Scholar]
- Peisach J., Blumberg W. E., Ogawa S., Rachmilewitz E. A., Oltzik R. The effects of protein conformation on the heme symmetry in high spin ferric heme proteins as studied by electron paramagnetic resonance. J Biol Chem. 1971 May 25;246(10):3342–3355. [PubMed] [Google Scholar]
- Vega J. M., Kamin H. Spinach nitrite reductase. Purification and properties of a siroheme-containing iron-sulfur enzyme. J Biol Chem. 1977 Feb 10;252(3):896–909. [PubMed] [Google Scholar]
- Zumft W. G. Ferredoxin:nitrite oxidoreductase from Chlorella. Purification and properties. Biochim Biophys Acta. 1972 Aug 28;276(2):363–375. doi: 10.1016/0005-2744(72)90996-5. [DOI] [PubMed] [Google Scholar]


