Abstract
1. The ethyl phosphotriester of thymidylyl(3'-5')thymidine, dTp(Et)dT, was identified as a product from reaction of DNA with N-ethyl-N-nitrosourea, by procedures parallel to those reported previously for the methyl homologue produced by N-methyl-N-nitrosourea. 2. Enzymic degradation to yield alkyl phosphotriesters from DNA alkylated by these carcinogens and by dimethyl sulphate and ethyl methanesulphonate was studied quantitatively, and the relative yields of the triesters dTp(Alk)dT were determined. The relative reactivity of the phosphodiester group dTpdT to each of the four carcinogens was thus obtained, and compared with that of DNA overall, or with that of the N-7 atom of guanine in DNA. Relative reactivity of the phosphodiester group was lowest towards dimethyl sulphate, the least electrophilic of the reagents used, and was highest towards N-ethyl-N-nitrosourea, the most electrophilic reagent. 3. The nature of the alkyl group transferred also influenced reactivity of the phosphodiester site, since this site was relatively more reactive towards ethylation than would be predicted simply from the known Swain-Scott s values of the alkylating agents. It was therefore suggested that the steric accessibility of the weakly nucleophilic phosphodiester group on the outside of the DNA macromolecule favours its reaction with ethylating, as opposed to methylating, reagents. 4. Taking a value of the Swain-Scott nucleophilicity (n) of 2.5 for an average DNA nucleotide unit [Walles & Ehrenberg (1969) Acta Chem. Scand. 23, 1080-1084], a value of n of about 1 for the phosphodiester group was deduced, and this value was found to be 2-3 units less than that for the N-7 atom of guanine in DNA. 5. The reactivity of DNA overall was markedly high towards the alkylnitrosoureas, despite their relatively low s values. This was ascribed to an electrostatic factor that favoured reaction of the negatively charged polymer with alkyldiazonium cation intermediates.
Full text
PDF


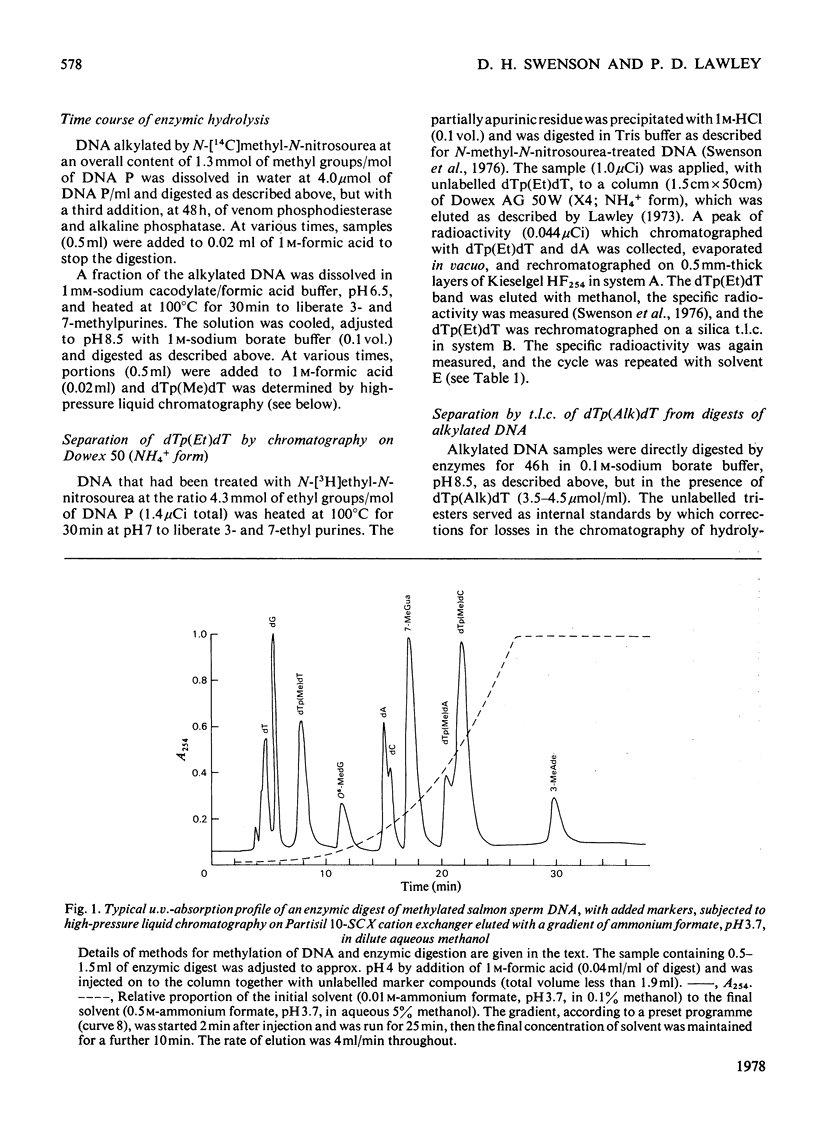
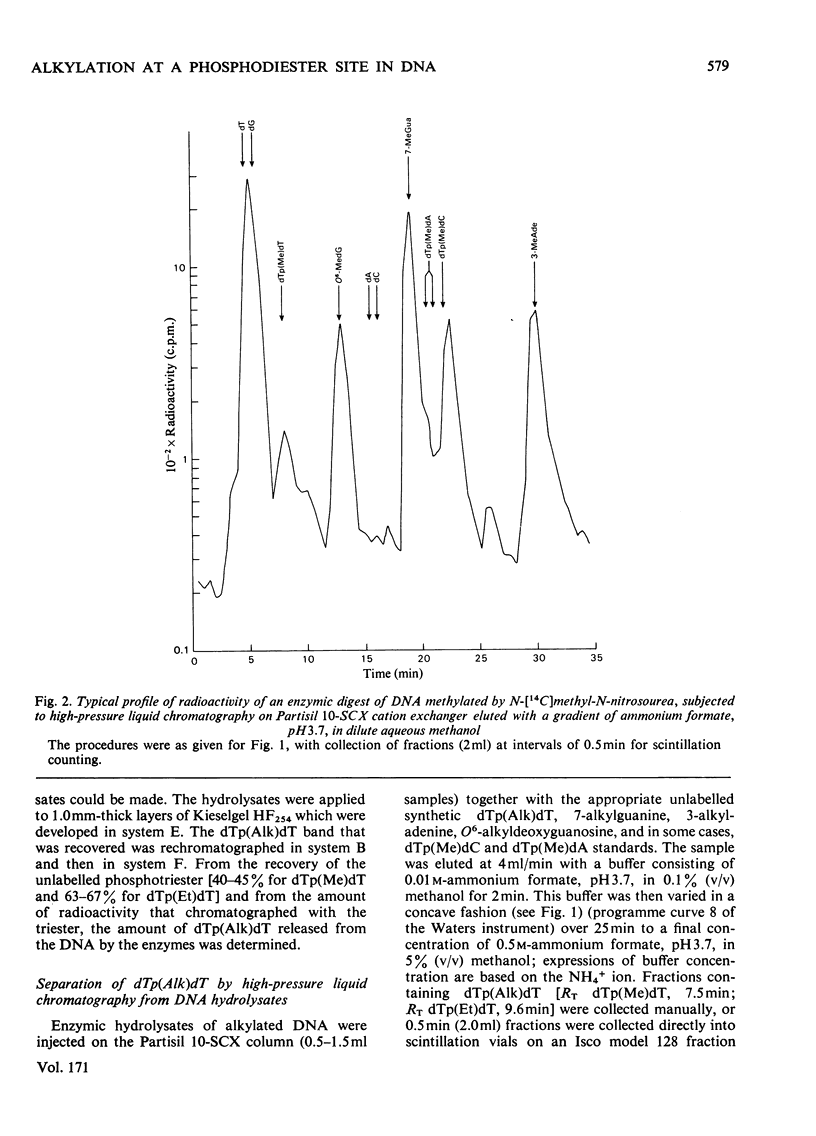
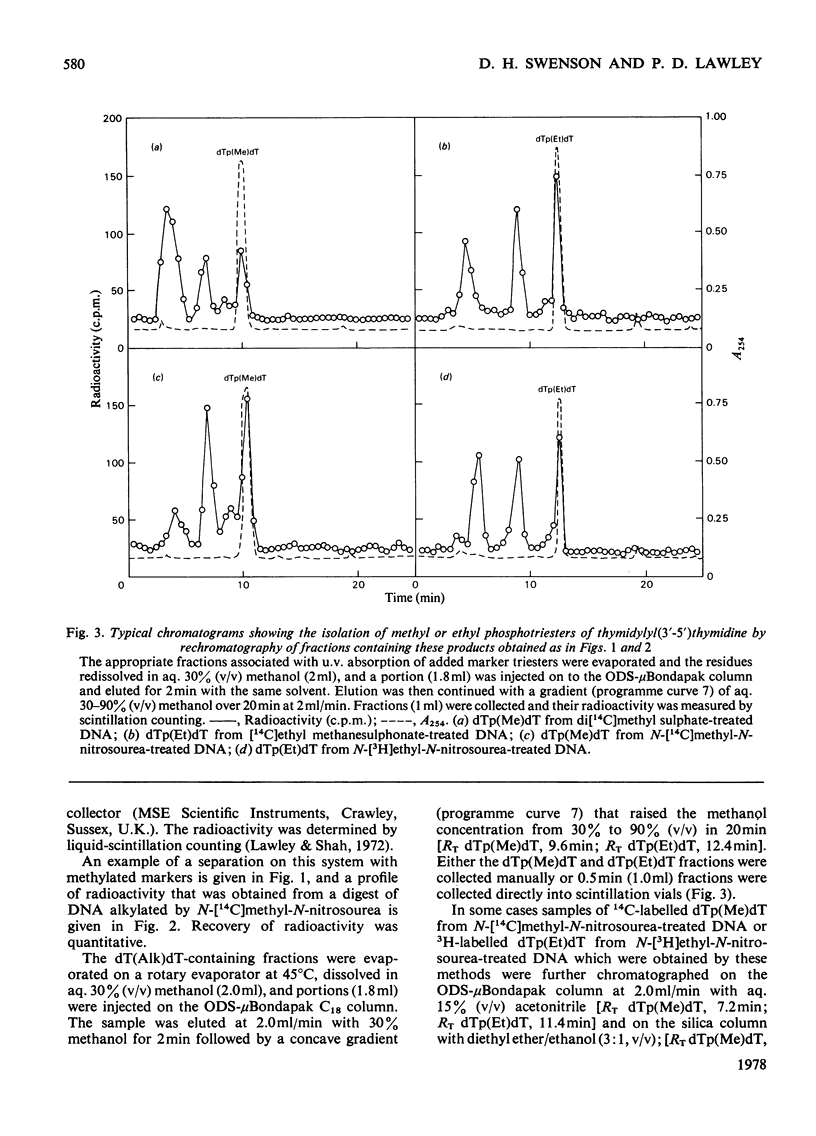
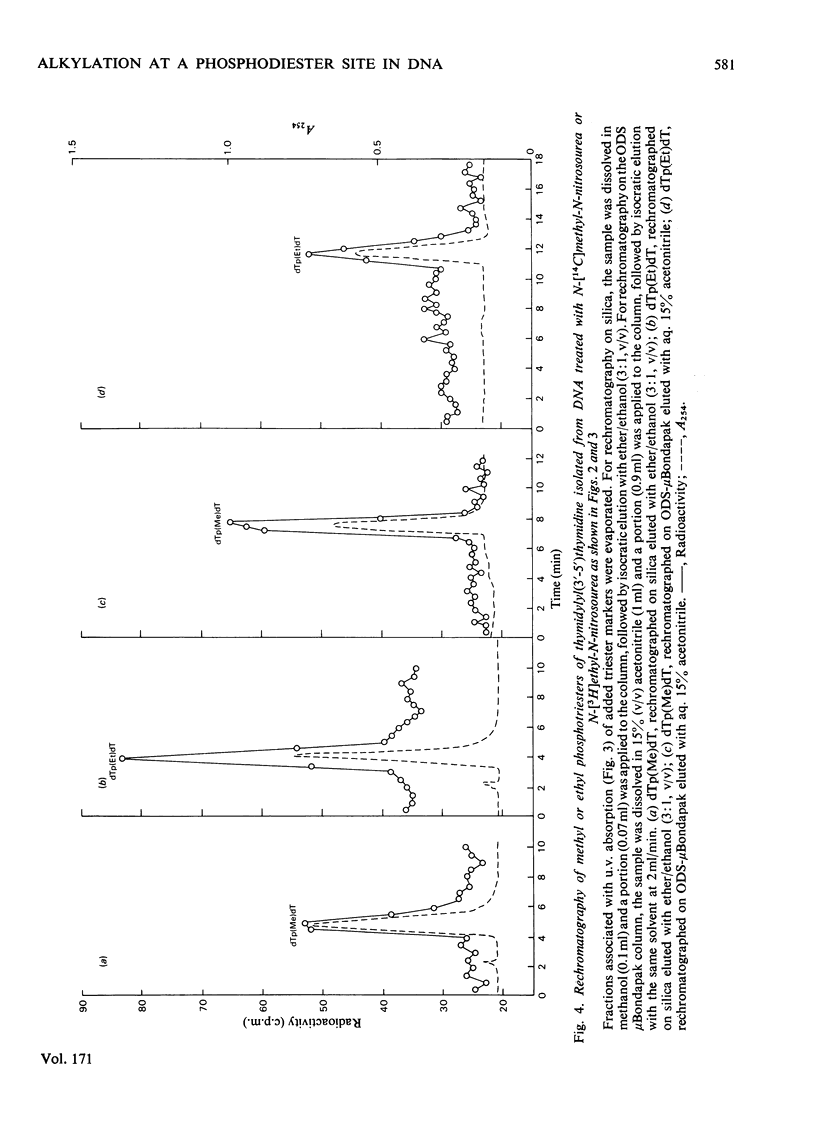





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bannon P., Verly W. Alkylation of phosphates and stability of phosphate triesters in DNA. Eur J Biochem. 1972 Nov 21;31(1):103–111. doi: 10.1111/j.1432-1033.1972.tb02506.x. [DOI] [PubMed] [Google Scholar]
- DeBoer G., Miller P. S., Ts'o P. O. Hydrogen-bonded complexes of adenine and thymine nucleoside alkyl phosphotriesters in deuteriochloroform. Biochemistry. 1973 Feb;12(4):720–726. doi: 10.1021/bi00728a023. [DOI] [PubMed] [Google Scholar]
- FELIX K., JILKE I., ZAHN R. K. Die Desoxyribonucleinsäure einiger Fischspermien. Hoppe Seylers Z Physiol Chem. 1956 Mar 17;303(3-6):140–152. [PubMed] [Google Scholar]
- Farmer P. B., Foster A. B., Jarman M., Tisdale M. J. The alkylation of 2'-deoxyguanosine and of thymidine with diazoalkanes. Some observations on o-alkylation. Biochem J. 1973 Sep;135(1):203–213. doi: 10.1042/bj1350203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawley P. D., Orr D. J., Jarman M. Isolation and identification of products from alkylation of nucleic acids: ethyl- and isopropyl-purines. Biochem J. 1975 Jan;145(1):73–84. doi: 10.1042/bj1450073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawley P. D., Orr D. J., Shah S. A., Farmer P. B., Jarman M. Reaction products from N-methyl-N-nitrosourea and deoxyribonucleic acid containing thymidine residues. Synthesis and identification of a new methylation product, O4-methylthymidine. Biochem J. 1973 Sep;135(1):193–201. doi: 10.1042/bj1350193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawley P. D. Reaction of N-methyl-N-nitrosourea (MNUA) with 32P-labelled DNA: evidence for formation of phosphotriesters. Chem Biol Interact. 1973 Aug;7(2):127–130. doi: 10.1016/0009-2797(73)90022-7. [DOI] [PubMed] [Google Scholar]
- Lawley P. D., Shah S. A. Methylation of ribonucleic acid by the carcinogens dimethyl sulphate, N-methyl-N-nitrosourea and N-methyl-N'-nitro-N-nitrosoguanidine. Comparisons of chemical analyses at the nucleoside and base levels. Biochem J. 1972 Jun;128(1):117–132. doi: 10.1042/bj1280117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawley P. D., Thatcher C. J. Methylation of deoxyribonucleic acid in cultured mammalian cells by N-methyl-N'-nitro-N-nitrosoguanidine. The influence of cellular thiol concentrations on the extent of methylation and the 6-oxygen atom of guanine as a site of methylation. Biochem J. 1970 Feb;116(4):693–707. doi: 10.1042/bj1160693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P. S., Barrett J. C., Ts'o P. O. Synthesis of oligodeoxyribonucleotide ethyl phosphotriesters and their specific complex formation with transfer ribonucleic acid. Biochemistry. 1974 Nov 19;13(24):4887–4896. doi: 10.1021/bi00721a003. [DOI] [PubMed] [Google Scholar]
- Miller P. S., Fang K. N., Kondo N. S., Ts'o P. O. Syntheses and properties of adenine and thymine nucleoside alkyl phosphotriesters, the neutral analogs of dinucleoside monophosphates. J Am Chem Soc. 1971 Dec;93(24):6657–6665. doi: 10.1021/ja00753a054. [DOI] [PubMed] [Google Scholar]
- O'Connor P. J., Marigison G. P., Craig A. W. Phosphotriesters in rat liver deoxyribonucleic acid after the administration of the carcinogen NN-dimethylnitrosamine in vivo. Biochem J. 1975 Mar;145(3):475–482. doi: 10.1042/bj1450475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shooter K. V., Merrifield R. K. An assay for phosphotriester formation in the reaction of alkylating agents with deoxyribosenucleic acid in vitro and in vivo. Chem Biol Interact. 1976 Jun;13(3-4):223–236. doi: 10.1016/0009-2797(76)90076-4. [DOI] [PubMed] [Google Scholar]
- Sun L., Singer B. The specificity of different classes of ethylating agents toward various sites of HeLa cell DNA in vitro and in vivo. Biochemistry. 1975 Apr 22;14(8):1795–1802. doi: 10.1021/bi00679a036. [DOI] [PubMed] [Google Scholar]
- Swenson D. H., Farmer P. B., Lawley P. D. Identification of the methyl phosphotriester of thymidylyl (3',5')thymidine as a product from reaction of DNA with the carcinogen N-methyl-N-nitrosourea. Chem Biol Interact. 1976 Sep;15(1):91–100. doi: 10.1016/0009-2797(76)90131-9. [DOI] [PubMed] [Google Scholar]
- Velemínský J., Osterman-Golkar S., Ehrenberg L. Reaction rates and biological action of N-methyl- and N-ethyl-N-nitrosourea. Mutat Res. 1970 Sep;10(3):169–174. doi: 10.1016/0027-5107(70)90113-2. [DOI] [PubMed] [Google Scholar]
- Walles S., Ehrenberg L. Determination of the rate constants for alkylation of DNA in vitro with methanesulfonic esters. Acta Chem Scand. 1969;23(3):1080–1082. doi: 10.3891/acta.chem.scand.23-1080. [DOI] [PubMed] [Google Scholar]


