Abstract
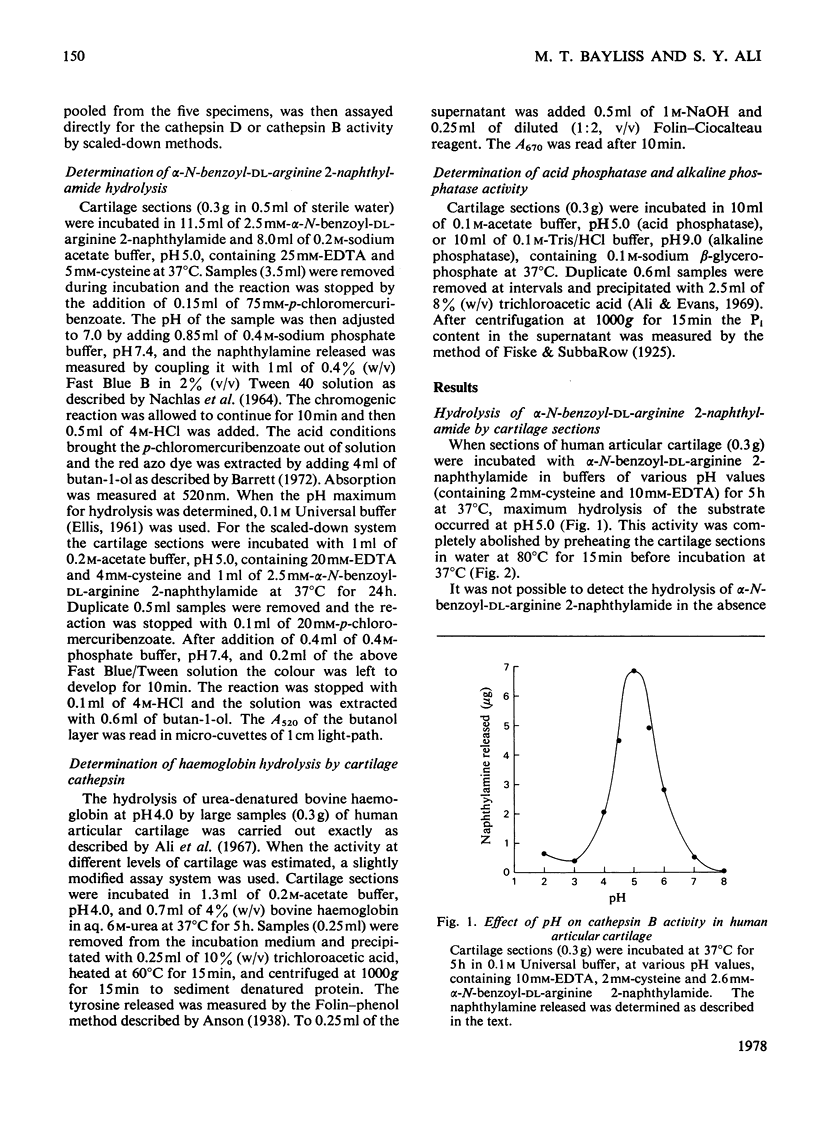
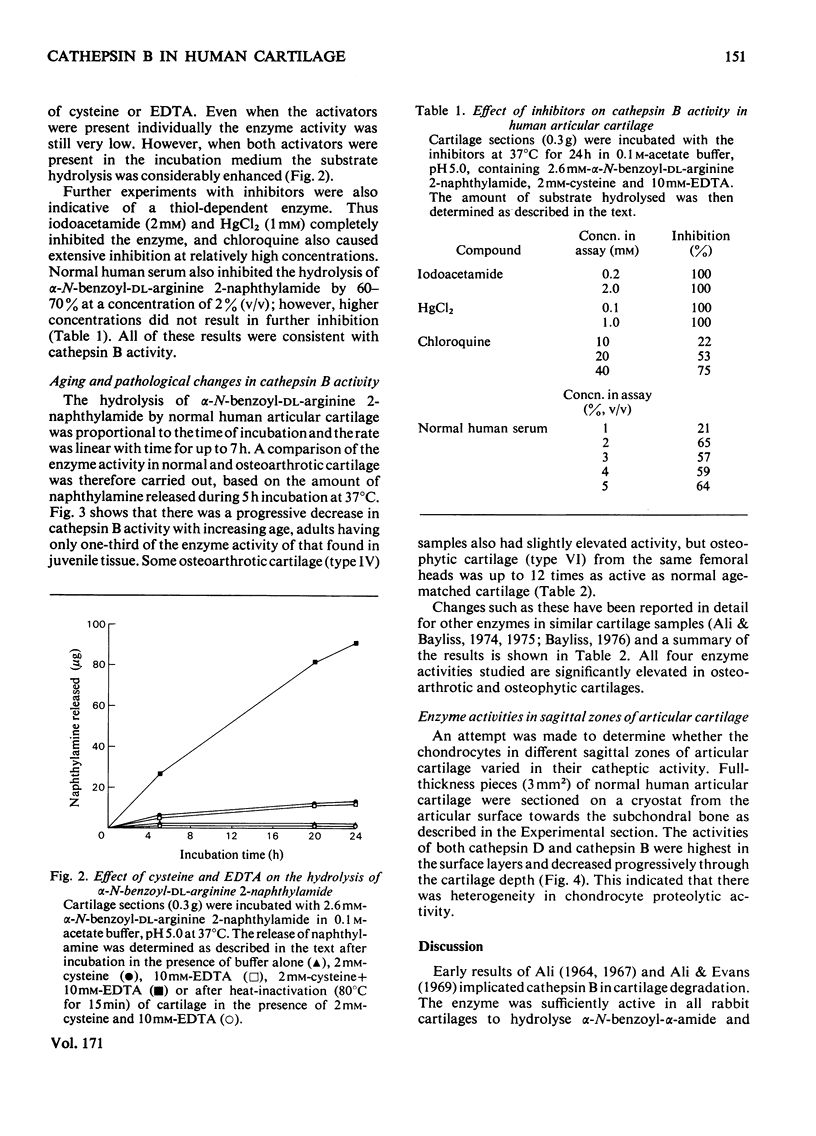
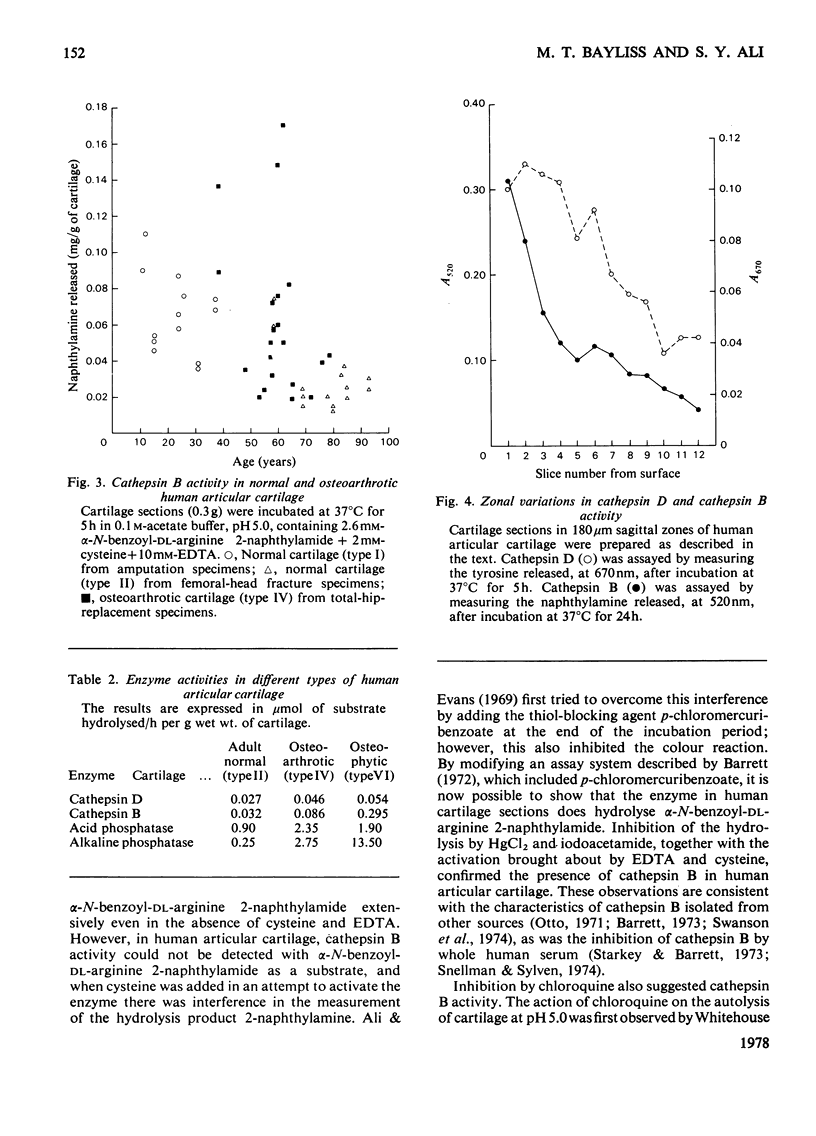
The thiol proteinase cathepsin B (EC 3.4.22.1), previously called cathepsin B1, was assayed in human articular cartilage by its hydrolysis of the synthetic substrate alpha-N-benzoyl-DL-arginine 2-naphthylamide. The enzyme was activated by cysteine and EDTA and completely inhibited by iodoacetamide and HgCl2. It was also partially inhibited by whole human serum. Human osteoarthrotic cartilage had increased activity when compared with normal cartilage. Cathepsin B activity of normal cartilage was age-related, being high in juveniles and declining to low values in adult and elderly individuals. Cathepsin D and cathepsin B both exhibited a zonal variation through the cartilage depth; the surface cells appeared to contain more activity than those close to the subchondral bone.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali S. Y., Evans L. Enzymic degradation of cartilage in osteoarthritis. Fed Proc. 1973 Apr;32(4):1494–1498. [PubMed] [Google Scholar]
- Ali S. Y., Evans L., Stainthorpe E., Lack C. H. Characterization of cathepsins in cartilage. Biochem J. 1967 Nov;105(2):549–557. doi: 10.1042/bj1050549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S. Y., Evans L. Studies on the cathepsins in elastic cartilage. Biochem J. 1969 May;112(4):427–433. doi: 10.1042/bj1120427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S. Y. The degradation of cartilage matrix by an intracellular protease. Biochem J. 1964 Dec;93(3):611–618. doi: 10.1042/bj0930611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J. A new assay for cathepsin B1 and other thiol proteinases. Anal Biochem. 1972 May;47(1):280–293. doi: 10.1016/0003-2697(72)90302-8. [DOI] [PubMed] [Google Scholar]
- Barrett A. J. Human cathepsin B1. Purification and some properties of the enzyme. Biochem J. 1973 Apr;131(4):809–822. doi: 10.1042/bj1310809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burleigh M. C., Barrett A. J., Lazarus G. S. Cathepsin B1. A lysosomal enzyme that degrades native collagen. Biochem J. 1974 Feb;137(2):387–398. doi: 10.1042/bj1370387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLLINS D. H., McELLIGOTT T. F. Sulphate (35SO4) uptake by chondrocytes in relation to histological changes in osteoarthritic human articular cartilage. Ann Rheum Dis. 1960 Dec;19:318–330. doi: 10.1136/ard.19.4.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingle J. T., Barrett A. J., Poole A. R., Stovin P. Inhibition by pepstatin of human cartilage degradation. Biochem J. 1972 Apr;127(2):443–444. doi: 10.1042/bj1270443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingle J. T., Barrett A. J., Weston P. D. Cathepsin D. Characteristics of immunoinhibition and the confirmation of a role in cartilage breakdown. Biochem J. 1971 Jun;123(1):1–13. doi: 10.1042/bj1230001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLIS D. A. A new universal buffer system. Nature. 1961 Sep 9;191:1099–1100. doi: 10.1038/1911099a0. [DOI] [PubMed] [Google Scholar]
- Etherington D. J. Bovine spleen cathepsin B1 and collagenolytic cathepsin. A comparative study of the properties of the two enzymes in the degradation of native collagen. Biochem J. 1976 Feb 1;153(2):199–209. doi: 10.1042/bj1530199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etherington D. J. The purification of bovine cathepsin B1 and its mode of action on bovine collagens. Biochem J. 1974 Mar;137(3):547–557. doi: 10.1042/bj1370547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans L., Sayers D. C., Ali S. Y. A method of preparation of cartilage for the study of its enzymes. J Med Lab Technol. 1967 Oct;24(4):306–308. [PubMed] [Google Scholar]
- Kincaid S. A., Van Sickle D. C., Wilsman N. J. Histochemical evidence of a functional heterogeneity of the chondrocytes of adult canine articular cartilage. Histochem J. 1972 May;4(3):237–243. doi: 10.1007/BF01890995. [DOI] [PubMed] [Google Scholar]
- LUCY J. A., DINGLE J. T., FELL H. B. Studies on the mode of action of excess of vitamin A. 2. A possible role of intracellular proteases in the degradation of cartilage matrix. Biochem J. 1961 Jun;79:500–508. doi: 10.1042/bj0790500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankin H. J., Lippiello L. The glycosaminoglycans of normal and arthritic cartilage. J Clin Invest. 1971 Aug;50(8):1712–1719. doi: 10.1172/JCI106660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroudas A. Glycosaminoglycan turn-over in articular cartilage. Philos Trans R Soc Lond B Biol Sci. 1975 Jul 17;271(912):293–313. doi: 10.1098/rstb.1975.0054. [DOI] [PubMed] [Google Scholar]
- Maroudas A., Muir H., Wingham J. The correlation of fixed negative charge with glycosaminoglycan content of human articular cartilage. Biochim Biophys Acta. 1969 May 6;177(3):492–500. doi: 10.1016/0304-4165(69)90311-0. [DOI] [PubMed] [Google Scholar]
- McDevitt C., Gilbertson E., Muir H. An experimental model of osteoarthritis; early morphological and biochemical changes. J Bone Joint Surg Br. 1977 Feb;59(1):24–35. doi: 10.1302/0301-620X.59B1.576611. [DOI] [PubMed] [Google Scholar]
- Morrison R. I., Barrett A. J., Dingle J. T., Prior D. Cathepsins BI and D. Action on human cartilage proteoglycans. Biochim Biophys Acta. 1973 Apr 12;302(2):411–419. doi: 10.1016/0005-2744(73)90170-8. [DOI] [PubMed] [Google Scholar]
- NACHLAS M. M., PLAPINGER R. E., SELIGMAN A. M. ROLE OF SOME STRUCTURAL FEATURES OF SUBTRATES ON TRYPSIN ACTIVITY. Arch Biochem Biophys. 1964 Nov;108:266–274. doi: 10.1016/0003-9861(64)90386-8. [DOI] [PubMed] [Google Scholar]
- Sapolsky A. I., Altman R. D., Woessner J. F., Howell D. S. The action of cathepsin D in human articular cartilage on proteoglycans. J Clin Invest. 1973 Mar;52(3):624–633. doi: 10.1172/JCI107224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky A. I., Howell D. S., Woessner J. F., Jr Neutral proteases and cathepsin D in human articular cartilage. J Clin Invest. 1974 Apr;53(4):1044–1053. doi: 10.1172/JCI107641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky A. I., Keiser H., Howell D. S., Woessner J. F., Jr Metalloproteases of human articular cartilage that digest cartilage proteoglycan at neutral and acid pH. J Clin Invest. 1976 Oct;58(4):1030–1041. doi: 10.1172/JCI108526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. E., Stockwell R. A. On the use and abuse of the critical electrolyte concentration approach to the localization of tissue polyanions. J Histochem Cytochem. 1967 Feb;15(2):111–113. doi: 10.1177/15.2.111. [DOI] [PubMed] [Google Scholar]
- Snellman O., Sylvén B. A carbohydrate inhibitor of cathepsin B activity associated with haptoglobin. Experientia. 1974 Oct 15;30(10):1114–1115. doi: 10.1007/BF01923639. [DOI] [PubMed] [Google Scholar]
- Starkey P. M., Barrett A. J. Inhibition by alpha-macroglobulin and other serum proteins. Biochem J. 1973 Apr;131(4):823–831. doi: 10.1042/bj1310823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockwell R. A., Scott J. E. Observations on the acid glycosaminoglycan (mucopolysaccharide) content of the matrix of aging cartilage. Ann Rheum Dis. 1965 Jul;24(4):341–350. doi: 10.1136/ard.24.4.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson A. A., Martin B. J., Spicer S. S. Human placental cathepsin B1. Isolation and some physical properties. Biochem J. 1974 Feb;137(2):223–228. doi: 10.1042/bj1370223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmann G. Lysosomes and joint disease. Arthritis Rheum. 1966 Dec;9(6):834–840. doi: 10.1002/art.1780090611. [DOI] [PubMed] [Google Scholar]
- Wibo M., Poole B. Protein degradation in cultured cells. II. The uptake of chloroquine by rat fibroblasts and the inhibition of cellular protein degradation and cathepsin B1. J Cell Biol. 1974 Nov;63(2 Pt 1):430–440. doi: 10.1083/jcb.63.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woessner J. F., Jr Cartilage cathepsin D and its action on matrix components. Fed Proc. 1973 Apr;32(4):1485–1488. [PubMed] [Google Scholar]
- Woessner J. F., Jr Purification of cathepsin D from cartilage and uterus and its action on the protein-polysaccharide complex of cartilage. J Biol Chem. 1973 Mar 10;248(5):1634–1642. [PubMed] [Google Scholar]


