Abstract
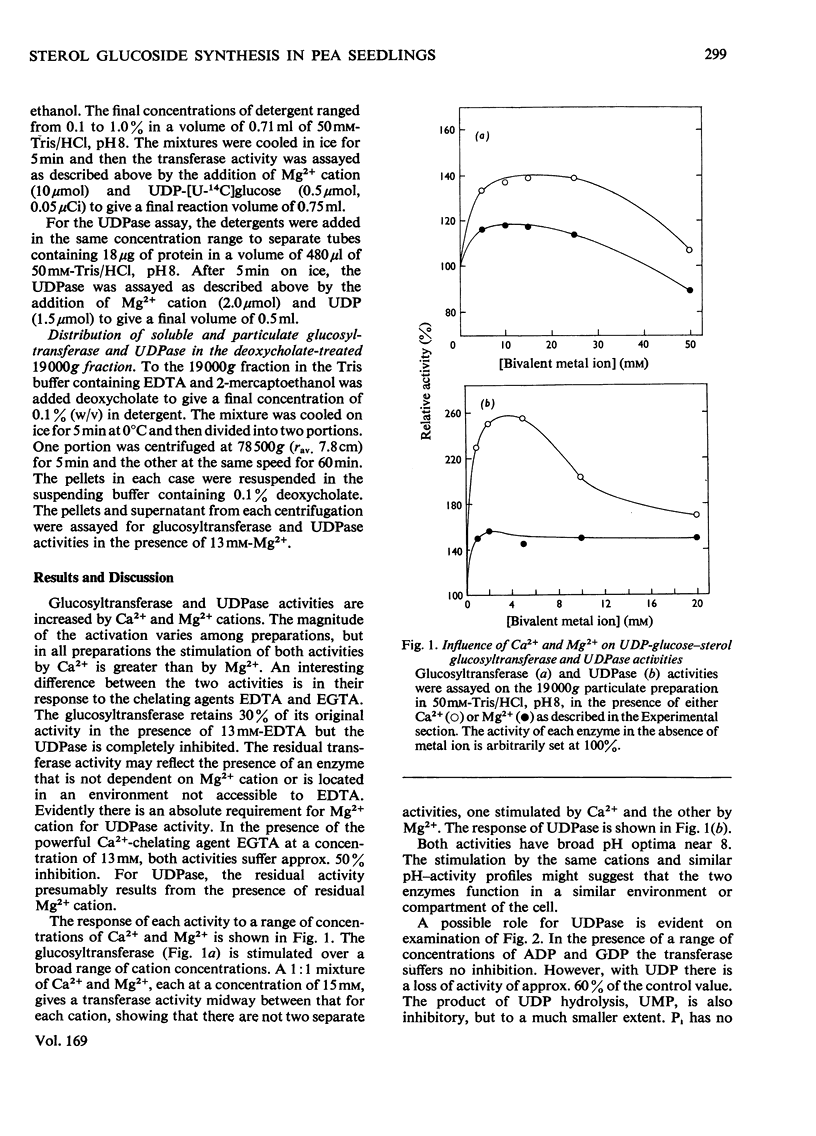
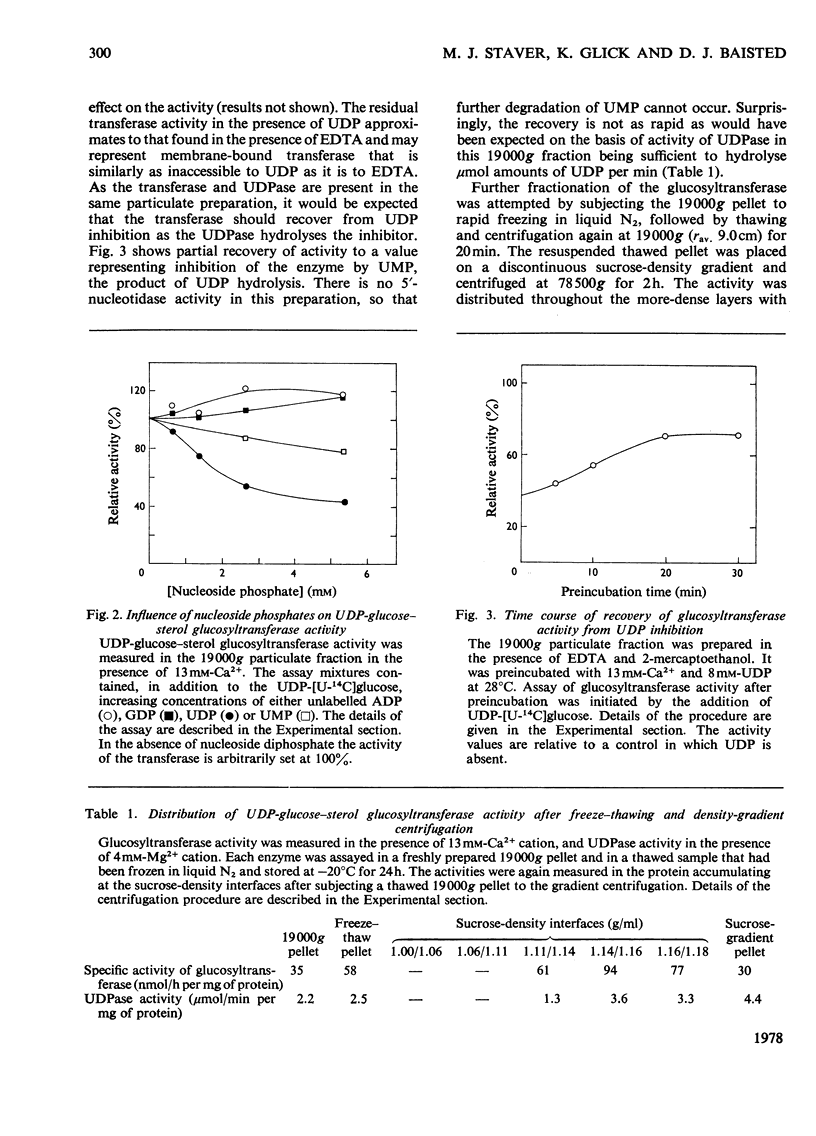
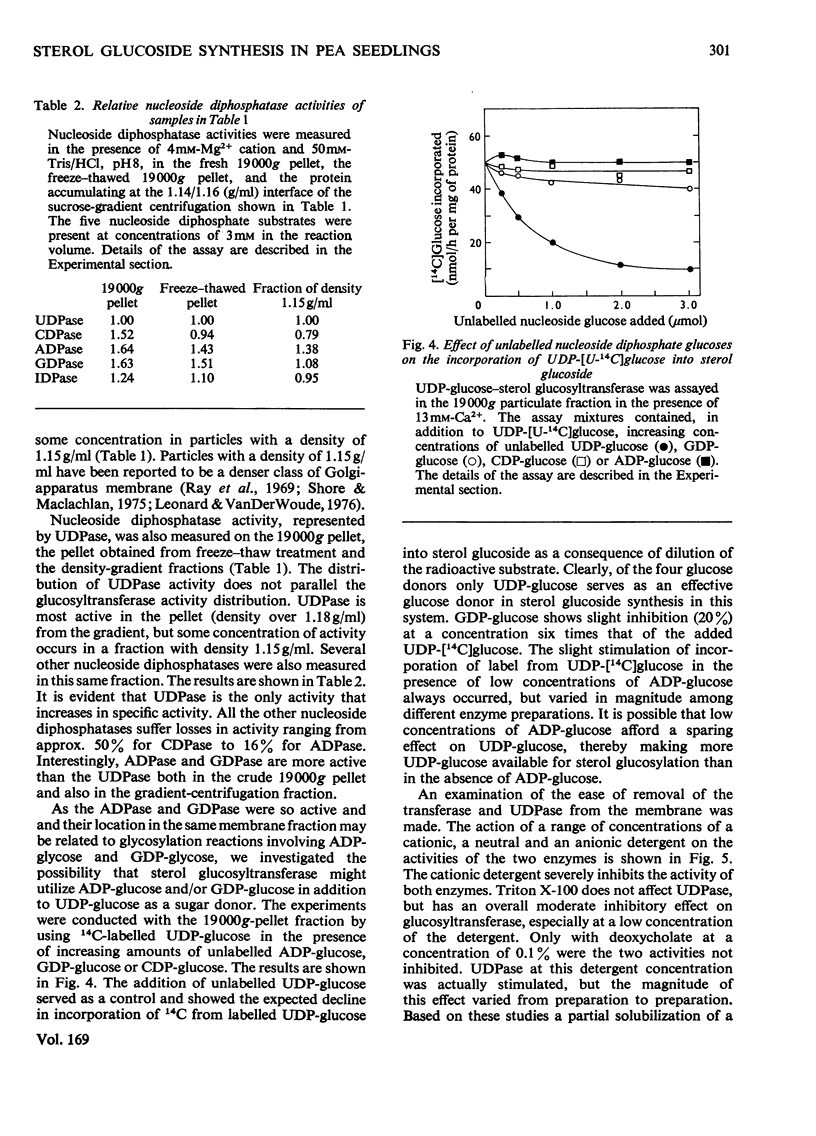
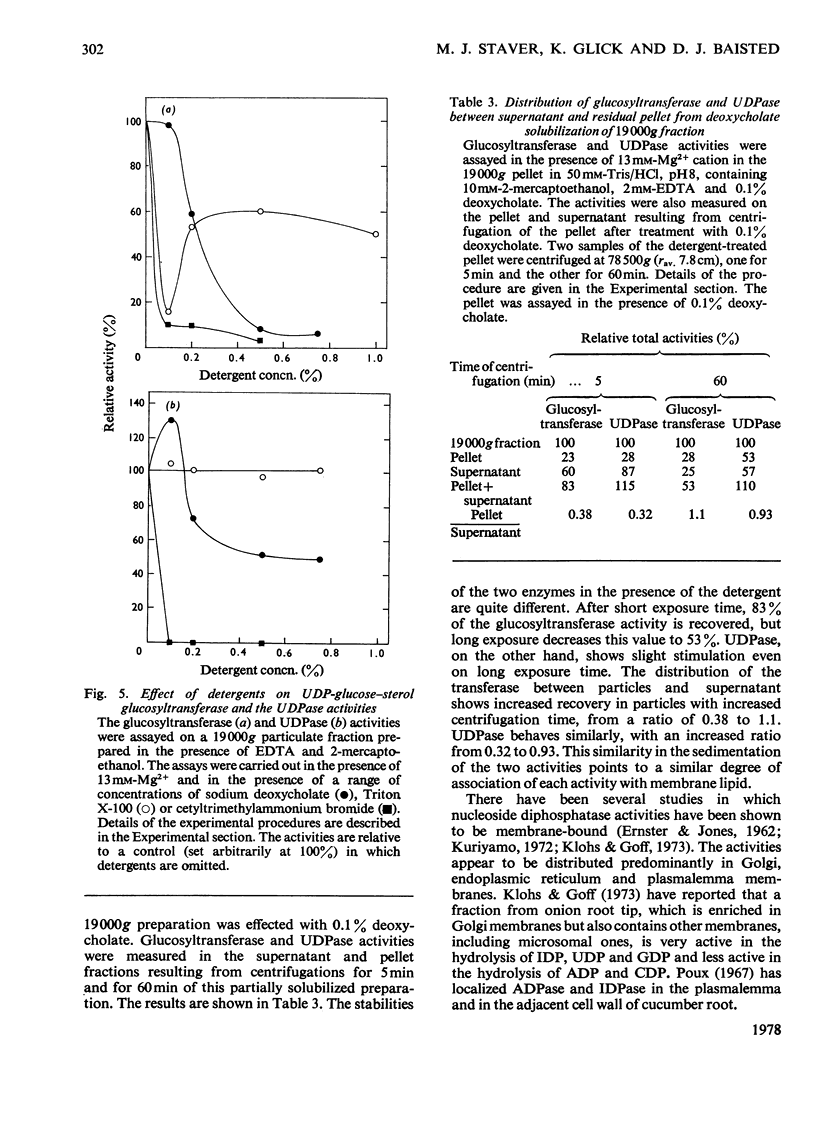
1. UDP-glucose-sterol glucosyltransferase and nucleoside diphosphatases were isolated in a particulate fraction from 7-day-old etiolated pea seedlings. The glucosyltransferase and UDPase (uridine diphosphatase) are stimulated by Ca2+ cation, less so by Mg2+ cation, and inhibited by Zn2+. 2. Each activity has a pH optimum near 8. 3. The glucosyltransferase is specific for UDP-glucose as the glucosyl donor and is inhibited by UDP. Partial recovery from UDP inhibition is effected by preincubation of the enzyme. 4. Freeze-thaw treatment and subsequent sucrose-density-gradient centrifugation of the particulate fraction shows the glucosyltransferase to be widely distributed among cell fractions but to be most active in particles with a density of 1.15 g/ml. UDPase is most active in particulate material with a density of over 1.18 g/ml but an activity peak also appears at 1.15 g/ml. Of several nucleoside diphosphatase activities, UDPase activity is most enhanced by the freeze-thaw and sucrose-density-gradient-fractionation procedures. 5. Detergent treatment with 0.1% sodium deoxycholate allows the partial solubilization of the glucosyltransferase and UDPase. The two activities are similarly distributed between pellet and supernatant after high-speed centrifugation for two different time intervals. 6. A role for UDPase in the functioning of glucosylation reactions is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bush P. B., Grunwald C. Steryl Glycoside Formation in Seedlings of Nicotiana tabacum L. Plant Physiol. 1974 Feb;53(2):131–135. doi: 10.1104/pp.53.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenberger W., Siegrist H. P. Steryl clycoside acyltransferase from carrots. FEBS Lett. 1975 Mar 15;52(1):153–156. doi: 10.1016/0014-5793(75)80660-0. [DOI] [PubMed] [Google Scholar]
- Goff C. W. Localization of nucleoside diphosphatase in the onion root tip. Protoplasma. 1973;78(4):397–416. doi: 10.1007/BF01275775. [DOI] [PubMed] [Google Scholar]
- Hou C. T., Umemura Y., Nakamura M., Funahashi S. Enzymatic synthesis of steryl glucoside by a particulate preparation from immature soybean seeds. J Biochem. 1968 Mar;63(3):351–360. [PubMed] [Google Scholar]
- Kauss H. Eznymatische Glucosylierung von pflanzlichen Sterinen. Z Naturforsch B. 1968 Nov;23(11):1522–1526. [PubMed] [Google Scholar]
- Klohs W. D., Goff C. W. A characterization of nucleoside diphosphatase in the onion root tip. J Histochem Cytochem. 1973 May;21(5):417–425. doi: 10.1177/21.5.417. [DOI] [PubMed] [Google Scholar]
- Kuriyama Y. Studies on microsomal nucleoside diphosphatase of rat hepatocytes. Its purification, intramembranous localization, and turnover. J Biol Chem. 1972 May 25;247(10):2979–2988. [PubMed] [Google Scholar]
- Laine R. A., Elbein A. D. Steryl glucosides in Phaseolus aureus. Use of gas-liquid chromatography and mass spectrometry for structural identification. Biochemistry. 1971 Jun 22;10(13):2547–2553. doi: 10.1021/bi00789a020. [DOI] [PubMed] [Google Scholar]
- Leonard R. T., Vanderwoude W. J. Isolation of plasma membranes from corn roots by sucrose density gradient centrifugation: an anomalous effect of ficoll. Plant Physiol. 1976 Jan;57(1):105–114. doi: 10.1104/pp.57.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezica R. P. Membrane-bound UDP-Glucose: Lipid Glucosyltransferases from Peas. Plant Physiol. 1976 Nov;58(5):675–680. doi: 10.1104/pp.58.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novikoff P. M., Novikoff A. B., Quintana N., Hauw J. J. Golgi apparatus, GERL, and lysosomes of neurons in rat dorsal root ganglia, studied by thick section and thin section cytochemistry. J Cell Biol. 1971 Sep;50(3):859–886. doi: 10.1083/jcb.50.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongun A., Mudd J. B. The biosynthesis of steryl glucosides in plants. Plant Physiol. 1970 Mar;45(3):255–262. doi: 10.1104/pp.45.3.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potty V. H. Determination of proteins in the presence of phenols and pectins. Anal Biochem. 1969 Jun;29(3):535–539. doi: 10.1016/0003-2697(69)90339-x. [DOI] [PubMed] [Google Scholar]
- Ray P. M., Shininger T. L., Ray M. M. ISOLATION OF beta-GLUCAN SYNTHETASE PARTICLES FROM PLANT CELLS AND IDENTIFICATION WITH GOLGI MEMBRANES. Proc Natl Acad Sci U S A. 1969 Oct;64(2):605–612. doi: 10.1073/pnas.64.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore G., Maclachlan G. A. The site of cellulose synthesis. Hormone treatment alters the intracellular location of alkali-insoluble beta-1,4-glucan (cellulose) synthetase activities. J Cell Biol. 1975 Mar;64(3):557–571. doi: 10.1083/jcb.64.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]


