Abstract
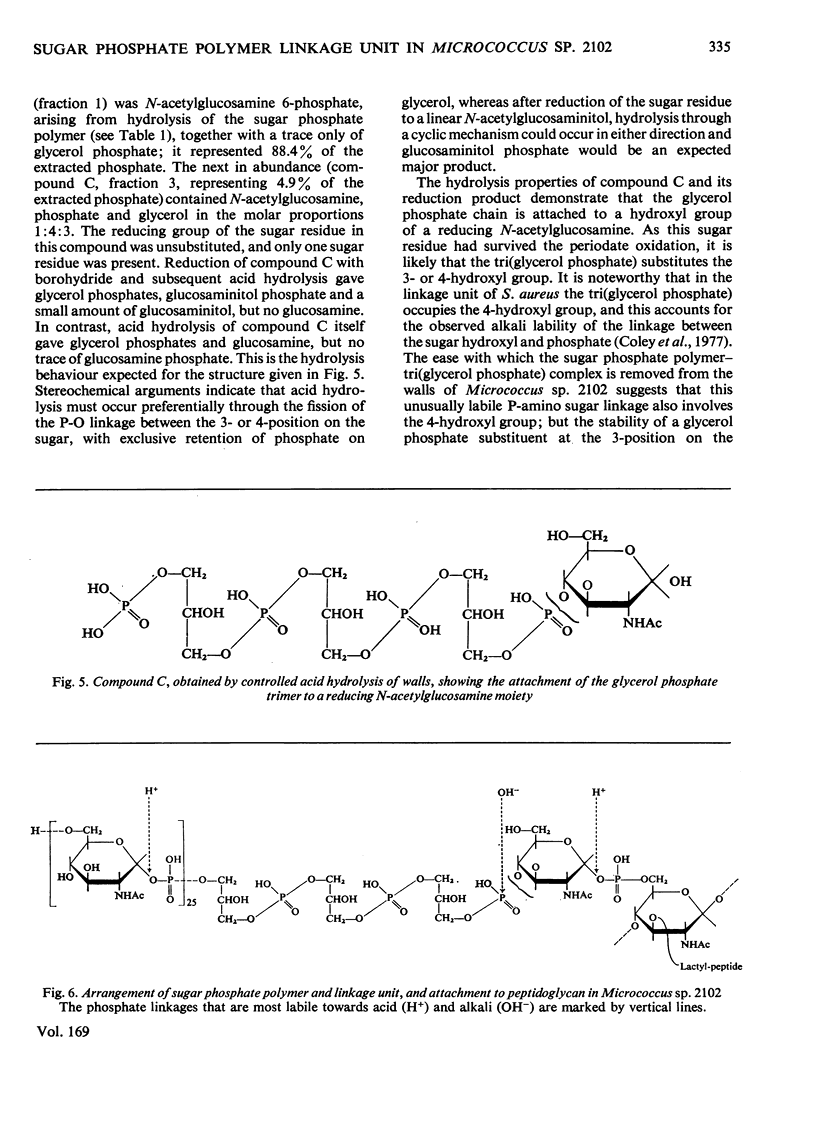
1. Protein-free walls of Micrococcus sp. 2102 contain peptidoglycan, poly-(N-acetylglucosamine 1-phosphate) and small amounts of glycerol phosphate. 2. After destruction of the poly-(N-acetylglucosamine 1-phosphate) with periodate, the glycerol phosphate remains attached to the wall, but can be removed by controlled alkaline hydrolysis. The homogeneous product comprises a chain of three glycerol phosphates and an additional phosphate residue. 3. The poly-(N-acetylglucosamine 1-phosphate) is attached through its terminal phosphate to one end of the tri(glycerol phosphate). 4. The other end of the glycerol phosphate trimer is attached through its terminal phosphate to the 3-or 4-position of an N-acetylglucosamine. It is concluded that the sequence of residues in the sugar 1-phosphate polymer-peptidoglycan complex is: (N-acetylglucosamine 1-phosphate)24-(glycerol phosphate)3-N-acetylglucosamine 1-phosphate-muramic acid (in peptidoglycan). Thus in this organism the phosphorylated wall polymer is attached to the peptidoglycan of the wall through a linkage unit comprising a chain of three glycerol phosphate residues and an N-acetylglucosamine 1-phosphate, similar to or identical with the linkage unit in Staphylococcus aureus H.
Full text
PDF

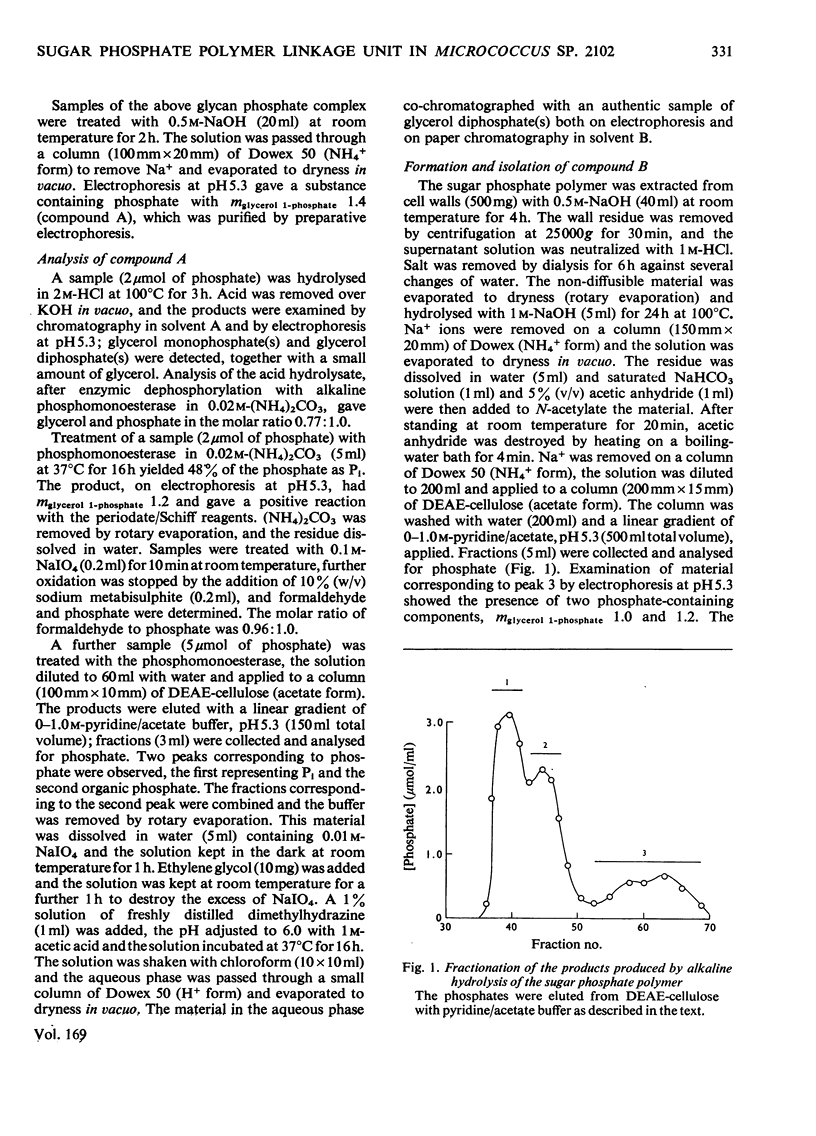
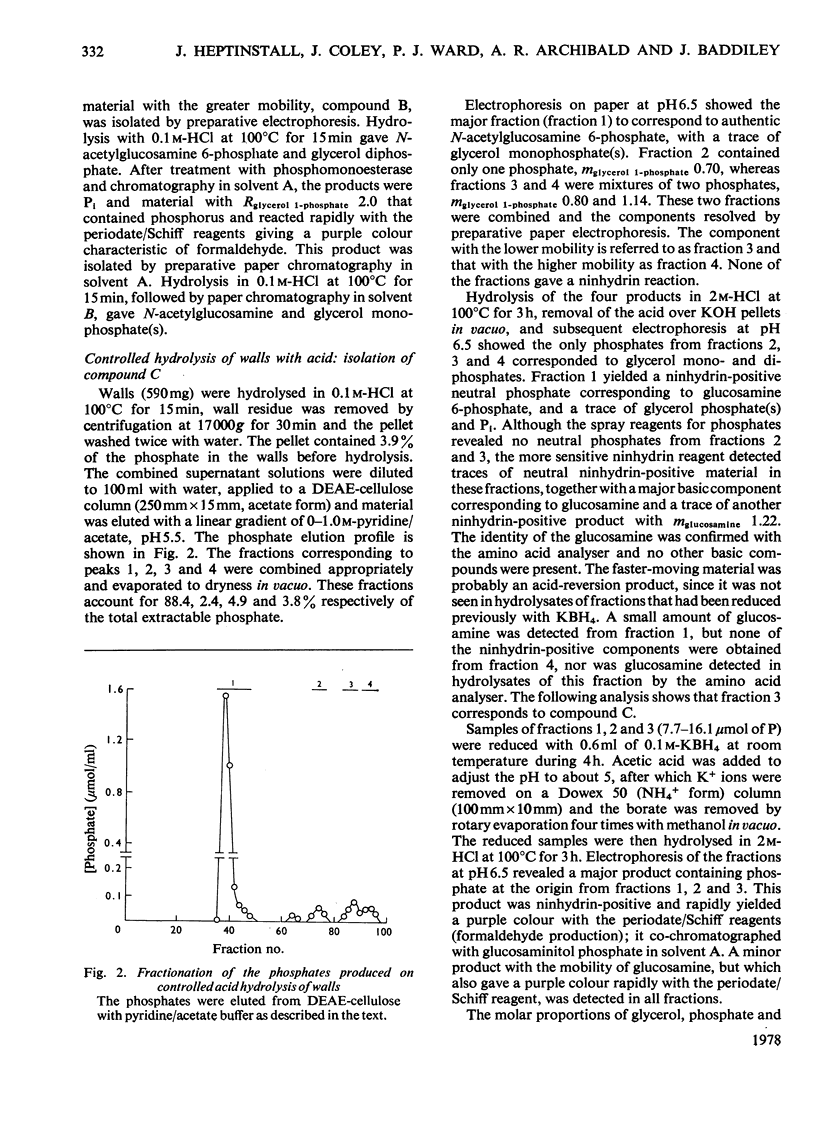
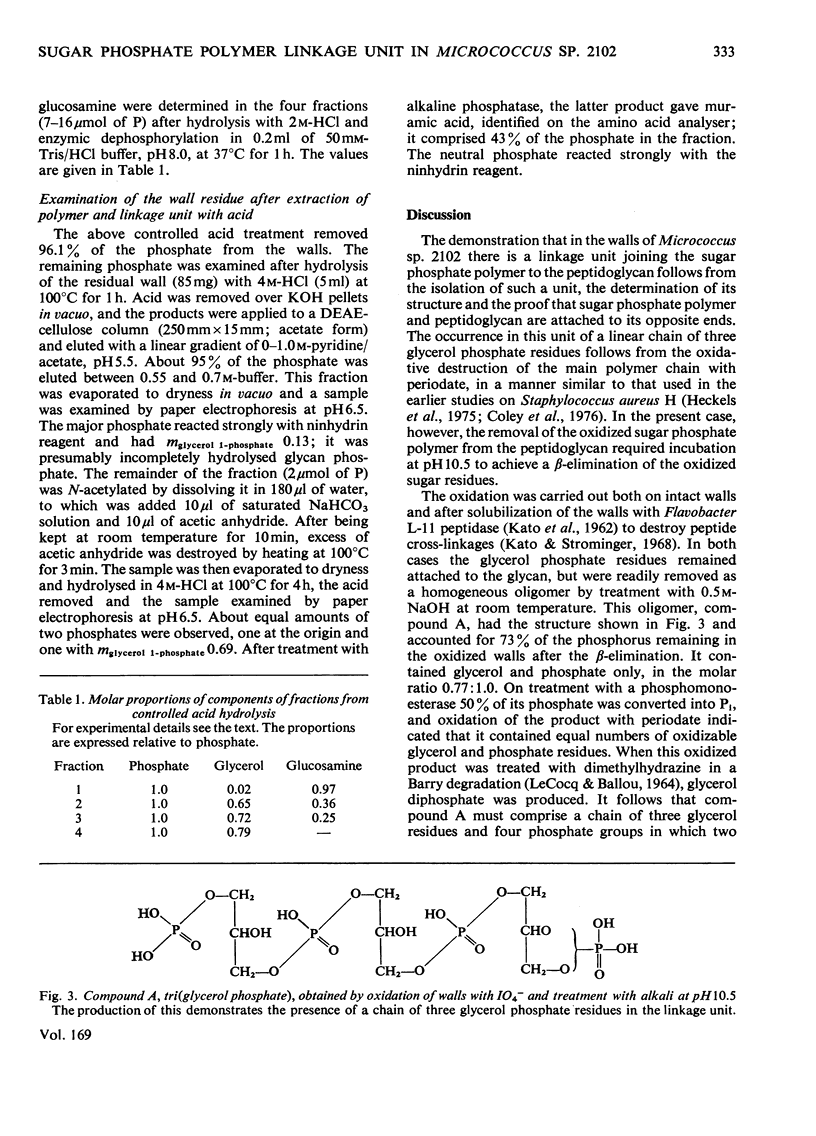
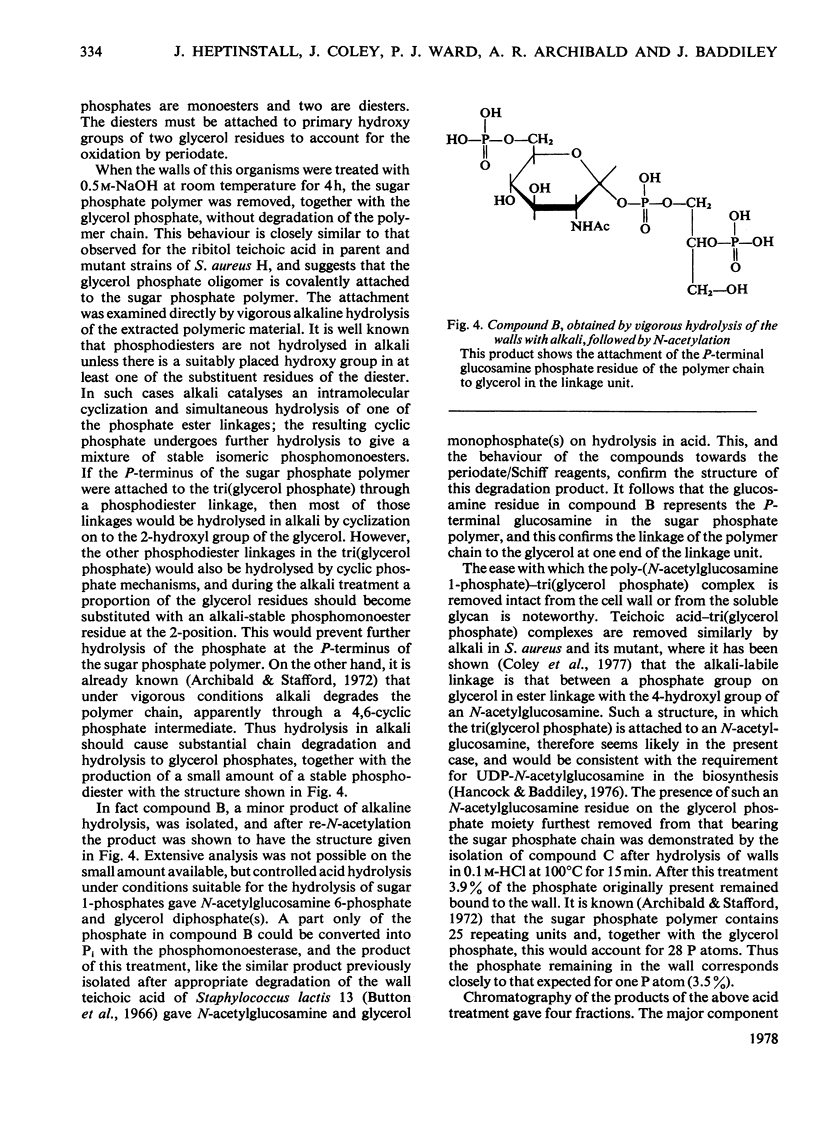

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archibald A. R., Stafford G. H. A polymer of N-acetylglucosamine 1-phosphate in the wall of Staphylococcus lactis 2102. Biochem J. 1972 Dec;130(3):681–690. doi: 10.1042/bj1300681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddiley J. Teichoic acids in cell walls and membranes of bacteria. Essays Biochem. 1972;8:35–77. [PubMed] [Google Scholar]
- Bracha R., Glaser L. An intermediate in telchoic acid biosynthesis. Biochem Biophys Res Commun. 1976 Oct 4;72(3):1091–1098. doi: 10.1016/s0006-291x(76)80244-6. [DOI] [PubMed] [Google Scholar]
- Coley J., Archibald A. R., Baddiley J. A linkage unit joining peptidoglycan to teichoic acid in Staphylococcus aureus H. FEBS Lett. 1976 Jan 15;61(2):240–242. doi: 10.1016/0014-5793(76)81047-2. [DOI] [PubMed] [Google Scholar]
- Coley J., Archibald R., Baddiley J. The presence of N-acetylglucosamine 1-phosphate in the linkage unit that connects teichoic acid to peptidoglycan in Staphylococcus aureus. FEBS Lett. 1977 Aug 15;80(2):405–407. doi: 10.1016/0014-5793(77)80486-9. [DOI] [PubMed] [Google Scholar]
- HANAHAN D. J., OLLEY J. N. Chemical nature of monophosphoinositides. J Biol Chem. 1958 Apr;231(2):813–828. [PubMed] [Google Scholar]
- HANES C. S., ISHERWOOD F. A. Separation of the phosphoric esters on the filter paper chromatogram. Nature. 1949 Dec 31;164(4183):1107-12, illust. doi: 10.1038/1641107a0. [DOI] [PubMed] [Google Scholar]
- Hancock I. C., Wiseman G., Baddiley J. Biosynthesis of the unit that links teichoic acid to the bacterial wall: inhibition by tunicamycin. FEBS Lett. 1976 Oct 15;69(1):75–80. doi: 10.1016/0014-5793(76)80657-6. [DOI] [PubMed] [Google Scholar]
- Hancock I., Baddiley J. In vitro synthesis of the unit that links teichoic acid to peptidoglycan. J Bacteriol. 1976 Mar;125(3):880–886. doi: 10.1128/jb.125.3.880-886.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckels J. E., Archibald A. R., Baddiley J. Studies on the linkage between teichoic acid and peptidoglycan in a bacteriophage-resistant mutant of Staphylococcus aureus H. Biochem J. 1975 Sep;149(3):637–647. doi: 10.1042/bj1490637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K., Strominger J. L. Structure of the cell wall of Staphylococcus aureaus. IX. Mechanism of hydrolysis by the L11 enzyme. Biochemistry. 1968 Aug;7(8):2745–2761. [PubMed] [Google Scholar]
- LECOCQ J., BALLOU C. E. ON THE STRUCTURE OF CARDIOLIPIN. Biochemistry. 1964 Jul;3:976–980. doi: 10.1021/bi00895a023. [DOI] [PubMed] [Google Scholar]
- Munoz E., Ghuysen J. M., Heymann H. Cell walls of Streptococcus pyogenes, type 14. C polysaccharide-peptidoglycan and G polysaccharide-peptidoglycan complexes. Biochemistry. 1967 Dec;6(12):3659–3670. doi: 10.1021/bi00864a007. [DOI] [PubMed] [Google Scholar]
- STROMINGER J. L., GHUYSEN J. M. ON THE LINKAGE BETWEEN TEICHOIC ACID AND THE GLYCOPEPTIDE IN THE CELL WALL OF STAPHYLOCOCCUS AUREUS. Biochem Biophys Res Commun. 1963 Aug 14;12:418–424. doi: 10.1016/0006-291x(63)90117-7. [DOI] [PubMed] [Google Scholar]
- Warner J. R., Morgan S. A., Shulman R. W. Kinetics of labeling of the S-adenosylmethionine pool of Saccharomyces cerevisiae. J Bacteriol. 1976 Mar;125(3):887–891. doi: 10.1128/jb.125.3.887-891.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyke A. W., Ward J. B. The biosynthesis of muramic acid phosphate in Bacillus licheniformis. FEBS Lett. 1977 Feb 1;73(2):159–163. doi: 10.1016/0014-5793(77)80971-x. [DOI] [PubMed] [Google Scholar]


