Abstract
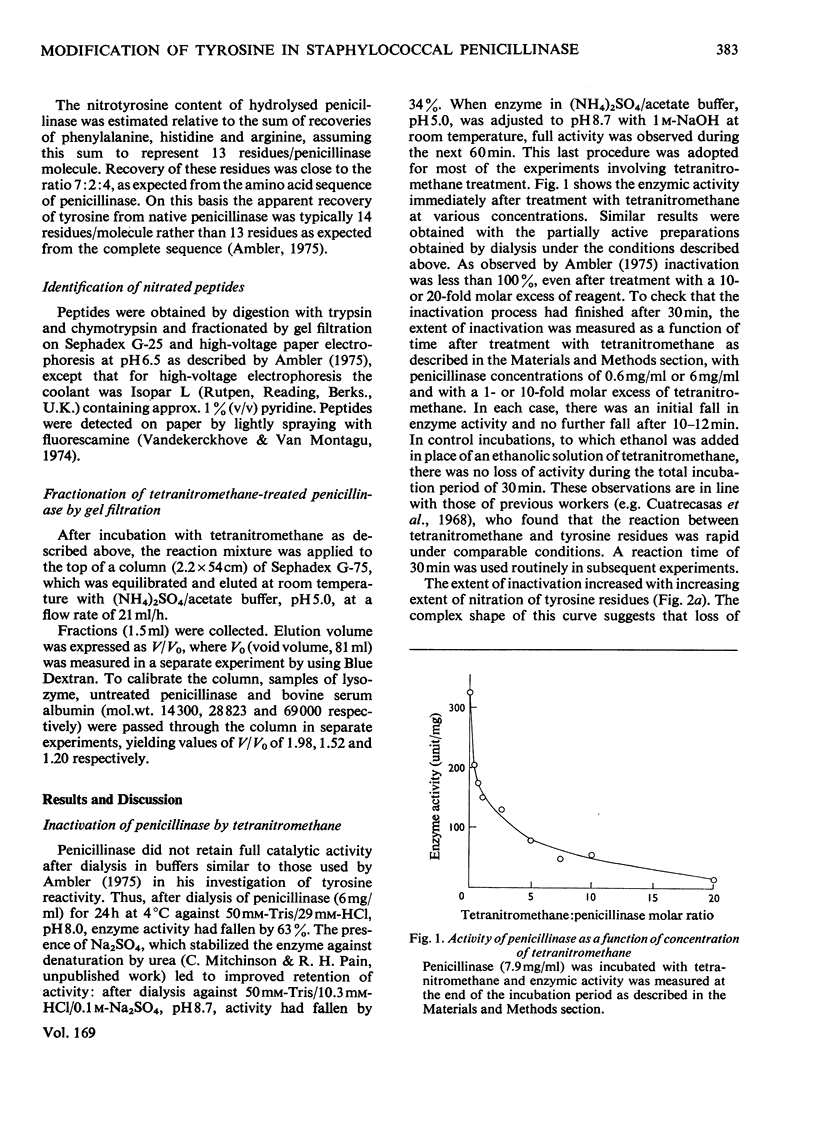
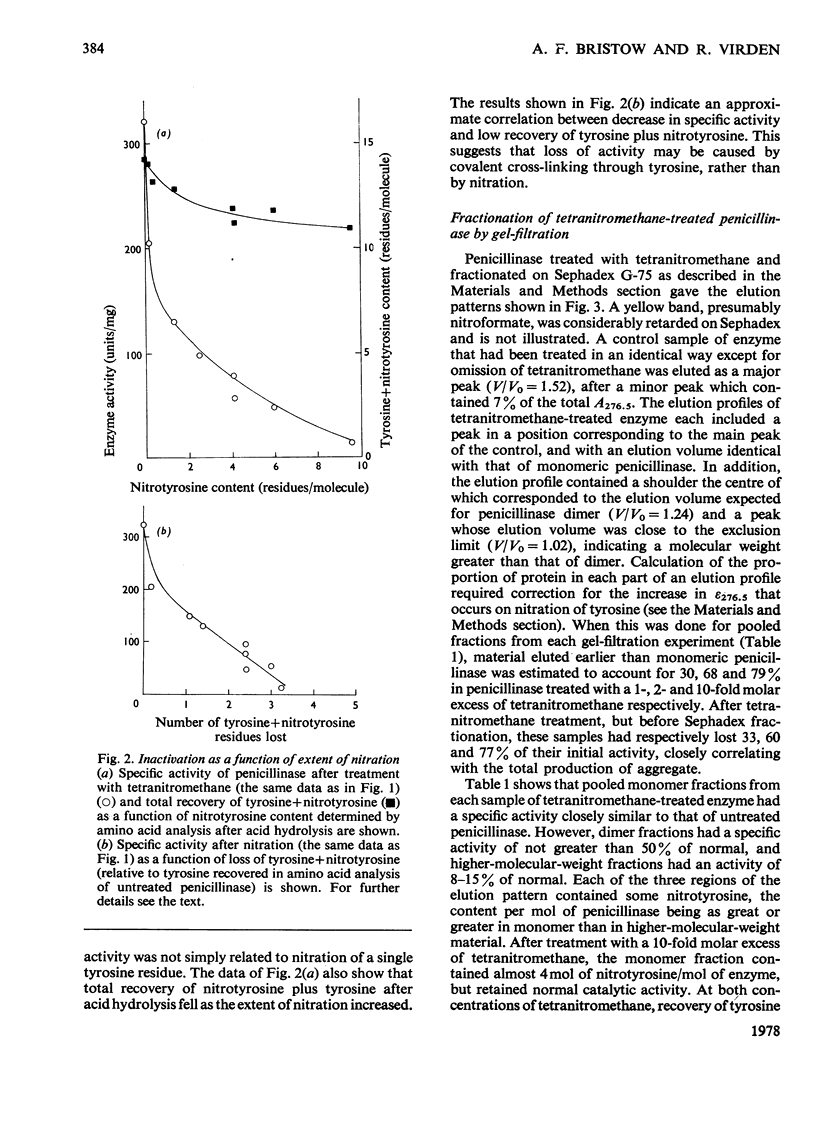
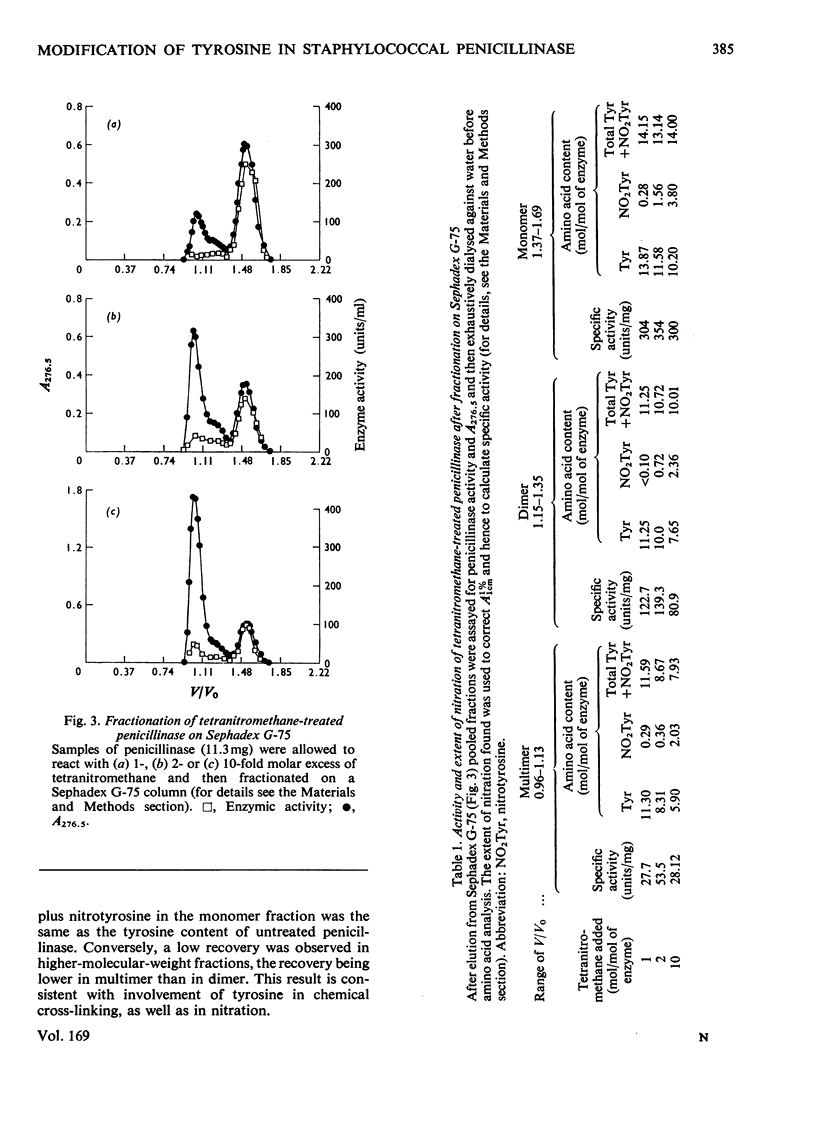
1. Nitration of tyrosine residues of staphylococal penicillinase was accompanied by a partial loss of enzymic activity, which was not readily explained by nitration of a single residue. 2. Loss of activity correlated with low recovery of tyrosine plus nitrotyrosine, which was consistent with cross-linking. 3. The fraction of treated enzyme that was eluted from Sephadex G-75 earlier than native penicillinase was similar to the fraction of enzyme activity lost. Protein eluted in positions corresponding to monomer, dimer and higher oligomers respectively showed major bands in corresponding positions in sodium dodecyl sulphate/polyacrylamide-gel electrophoresis, indicating that the increase in molecular weight was due to intermolecular cross-linking. Monomeric enzyme containing up to 4 mol of nitrotyrosine/mol retained full catalytic activity. Dimeric enzyme retained 50% of normal activity, whereas higher oligomers retained an average of 8-15% of normal activity. 4. Monomeric enzyme isolated after treatment with equimolar tetranitromethane was nitrated predominantly at tyrosine-72.5. Reaction of reduced nitrated monomer with 1,5-difluoro-2,4-dinitrobenzene gave a monomeric, apparently cross-linked product with full catalytic activity. 6. It is concluded that tyrosine-72 plays no part in the active site. Its preferential nitration may be due to its being insufficiently exposed to be available for intermolecular cross-linking. This poperty may make it useful for attachment of a reporter group.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambler R. P. The amino acid sequence of Staphylococcus aureus penicillinase. Biochem J. 1975 Nov;151(2):197–218. doi: 10.1042/bj1510197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesel R. W., Carpenter F. H. Crosslinking during the nitration of bovine insulin with tetranitromethane. Biochem Biophys Res Commun. 1970 Feb 20;38(4):678–682. doi: 10.1016/0006-291x(70)90634-0. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P., Fuchs S., Anfinsen C. B. Cross-linking of aminotyrosyl residues in the active site of staphylococcal nuclease. J Biol Chem. 1969 Jan 25;244(2):406–412. [PubMed] [Google Scholar]
- Cuatrecasas P., Fuchs S., Anfinsen C. B. The tyrosyl residues at the active site of staphylococcal nuclease. Modifications by tetranitromethane. J Biol Chem. 1968 Sep 25;243(18):4787–4798. [PubMed] [Google Scholar]
- Doyle R. J., Bello J., Roholt O. A. Probable protein crosslinking with tetranitromethane. Biochim Biophys Acta. 1968 Jun 26;160(2):274–276. doi: 10.1016/0005-2795(68)90103-7. [DOI] [PubMed] [Google Scholar]
- Fawcett R. I., Limbird T. J., Oliver S. L., Borders C. L., Jr Chemical modification of human lysozyme. Acetylation and nitration of tyrosine. Can J Biochem. 1971 Jul;49(7):816–821. doi: 10.1139/o71-115. [DOI] [PubMed] [Google Scholar]
- RICHMOND M. H. PURIFICATION AND PROPERTIES OF THE EXOPENICILLINASE FROM STAPHYLOCOCCUS AUREUS. Biochem J. 1963 Sep;88:452–459. doi: 10.1042/bj0880452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riordan J. F., Sokolovsky M., Vallee B. L. Environmentally sensitive tyrosyl residues. Nitration with tetranitromethane. Biochemistry. 1967 Jan;6(1):358–361. doi: 10.1021/bi00853a053. [DOI] [PubMed] [Google Scholar]
- Robson B., Pain R. H. The mechanism of folding of globular proteins. Suitability of a penicillinase from Staphylococcus Aureus as a model for refolding studies. Biochem J. 1976 May 1;155(2):325–330. doi: 10.1042/bj1550325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANGER F., THOMPSON E. O. Halogenation of tyrosine during acid hydrolysis. Biochim Biophys Acta. 1963 May 14;71:468–471. doi: 10.1016/0006-3002(63)91108-9. [DOI] [PubMed] [Google Scholar]
- Shifrin S., Solis B. G. L-asparaginase from Escherichia coli B. Chemical modifications of tyrosyl residues. J Biol Chem. 1972 Jul 10;247(13):4121–4125. [PubMed] [Google Scholar]
- Shih C. Y., Craven G. R. Identification of neighbor relationships among proteins in the 30 S ribosome: intermolecular cross-linkage of three proteins induced by tetranitromethane. J Mol Biol. 1973 Aug 25;78(4):651–663. doi: 10.1016/0022-2836(73)90286-6. [DOI] [PubMed] [Google Scholar]
- Sokolovsky M., Harell D., Riordan J. F. Reaction of tetranitromethane with sulfhydryl groups in proteins. Biochemistry. 1969 Dec;8(12):4740–4745. doi: 10.1021/bi00840a013. [DOI] [PubMed] [Google Scholar]
- Tsukamoto S., Ono M. Polymerization of papain by the reaction of its tyrosine residues with tetranitromethane. J Biochem. 1974 Jun;75(6):1377–1380. doi: 10.1093/oxfordjournals.jbchem.a130524. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove J., Van Montagu M. Sequence analysis of fluorescamine-stained peptides and proteins purified on a nanomole scale. Application to proteins of bacteriophage MS2. Eur J Biochem. 1974 May 2;44(1):279–288. doi: 10.1111/j.1432-1033.1974.tb03483.x. [DOI] [PubMed] [Google Scholar]
- Vincent J. P., Lazdunski M., Delaage M. On the use of tetranitromethane as a nitration reagent. The reaction of phenol side-chains in bovine and porcine trypsinogens and trypsins. Eur J Biochem. 1970 Feb;12(2):250–257. doi: 10.1111/j.1432-1033.1970.tb00844.x. [DOI] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]
- Williams J., Lowe J. M. The cross-linking of tyrosine by treatment with tetranitromethane. Biochem J. 1971 Jan;121(2):203–209. doi: 10.1042/bj1210203. [DOI] [PMC free article] [PubMed] [Google Scholar]


