Abstract
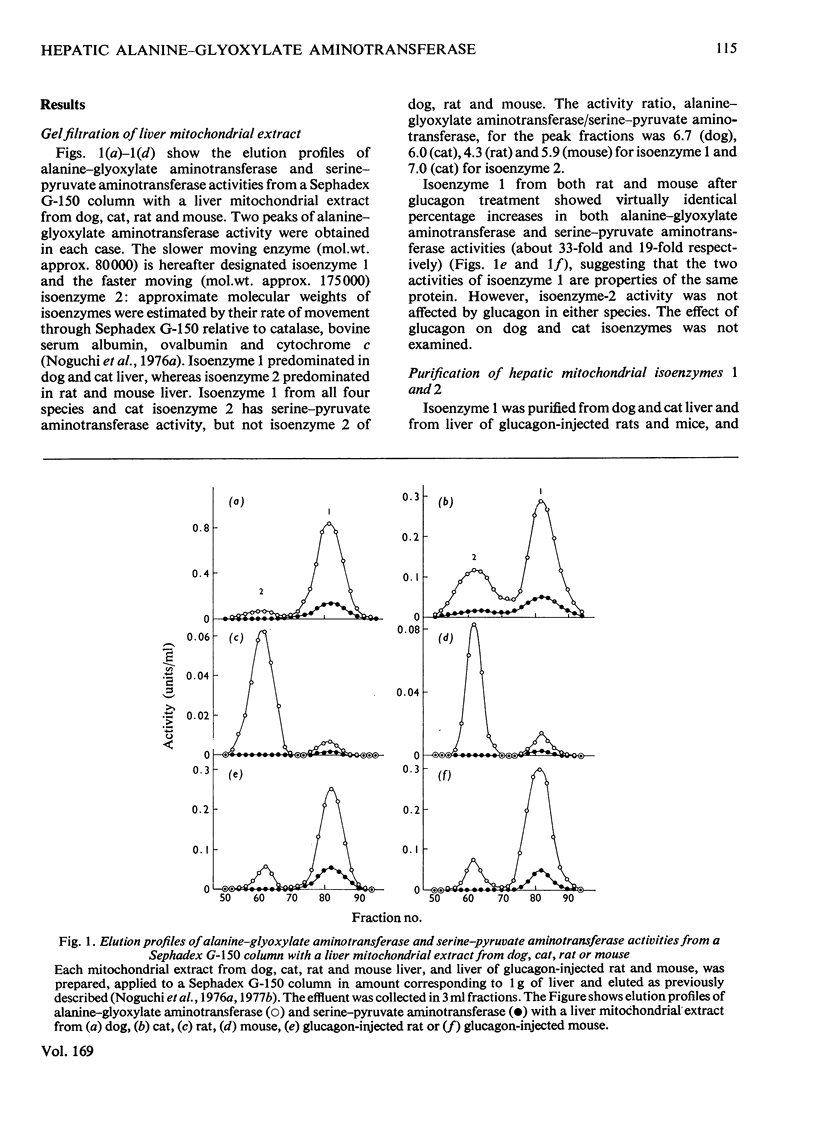
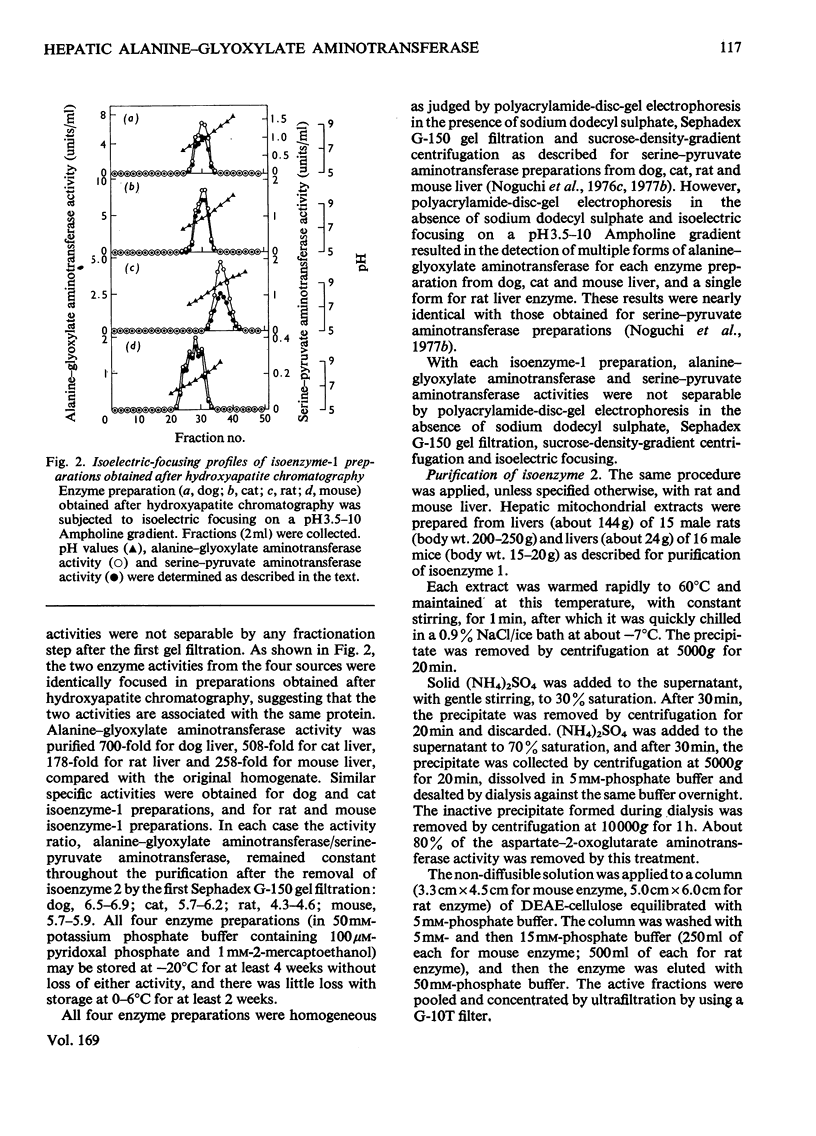
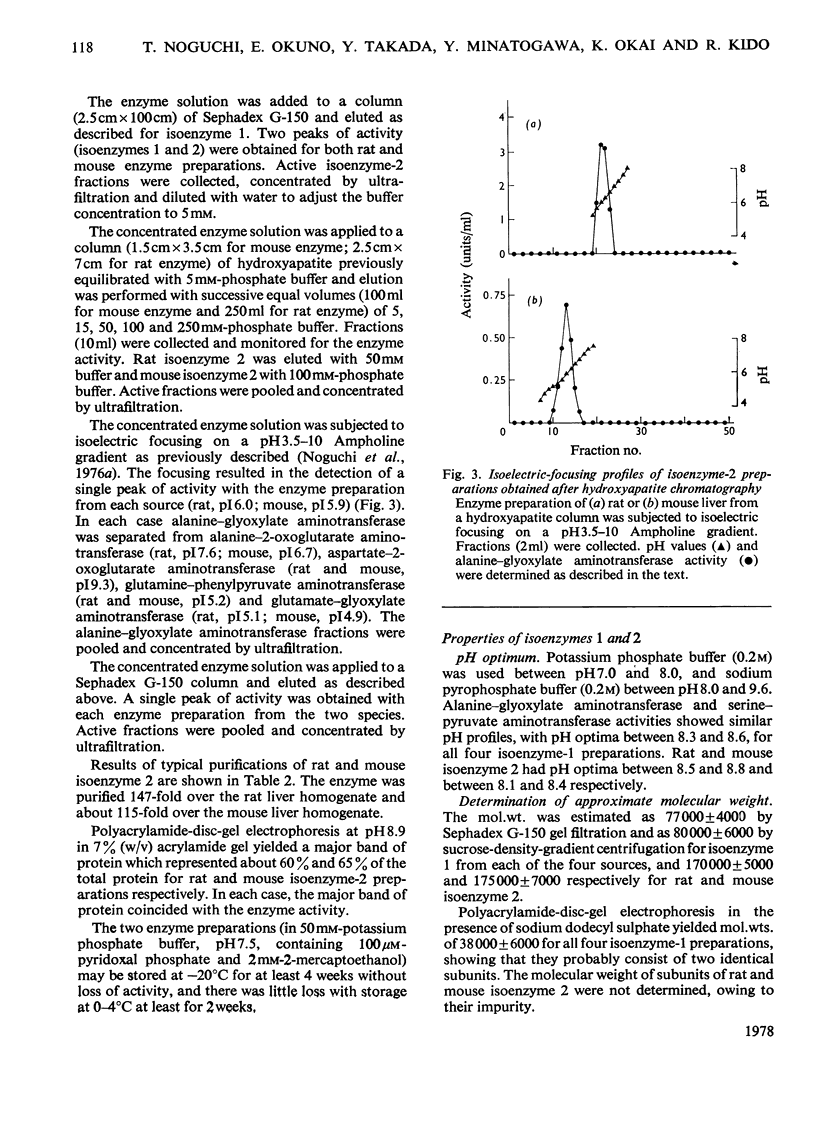
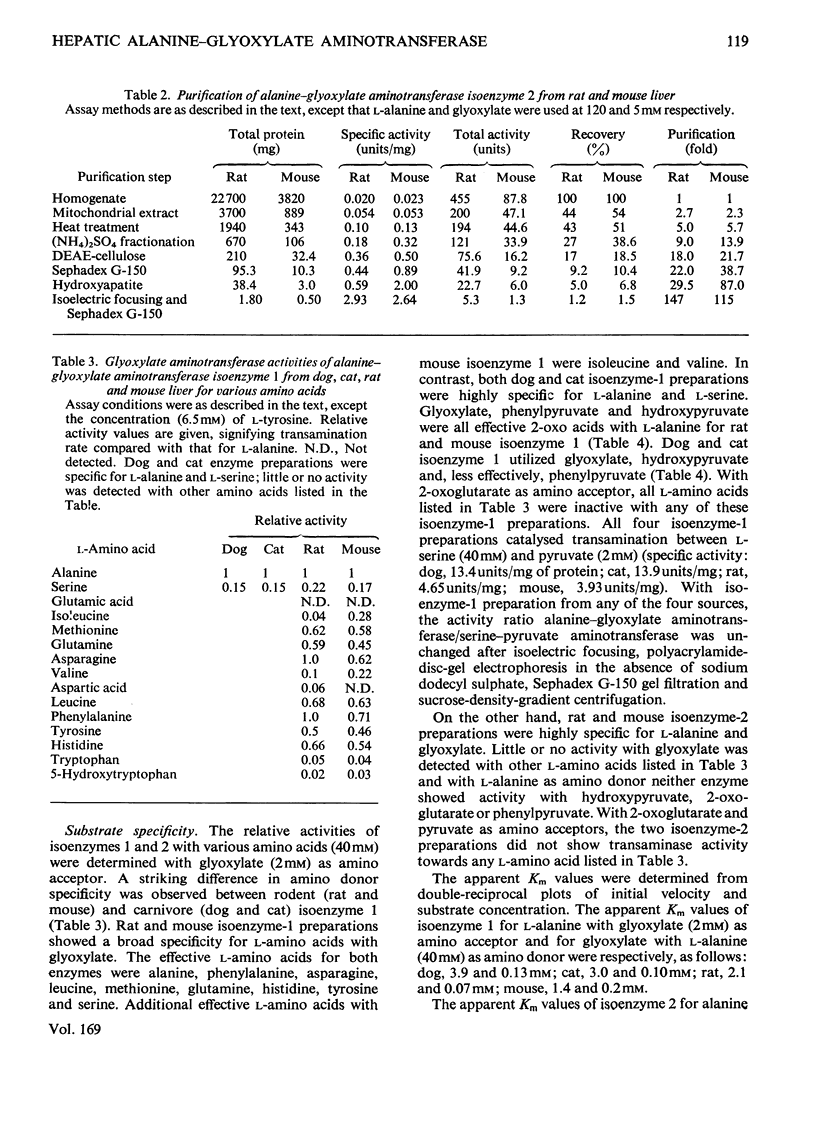
Mitochondrial extracts of dog, cat, rat and mouse liver contain two forms of alanine-glyoxylate aminotransferase (EC 2.6.1.44): one, designated isoenzyme 1, has mol.wt. approx. 80 000 and predominates in dog and cat liver; the other, designated isoenzyme 2, has mol.wt. approx. 175 000 and predominates in rat and mouse liver. In rat and mouse liver, isoenzyme 1 activity was increased by the injection in vivo of glucagon, but not isoenzyme 2 activity. Isoenzyme 1 was purified and characterized from liver mitochondrial extracts of the four species. Both rat and mouse enzyme preparations catalysed transamination between a number of L-amino acids and glyoxylate, and with L-alanine as amino donor the effective amino acceptors were glyoxylate, phenylpyruvate and hydroxypyruvate. In contrast, both dog and cat enzyme preparations were specific for L-alanine and L-serine with glyoxylate, and used glyoxylate and hydroxypyruvate as effective amino acceptors with L-alanine. Evidence that isoenzyme 1 is identical with serine-pyruvate aminotransferase (EC 2.6.1.51) was obtained. Isoenzyme 2 was partially purified from mitochondrial extracts of rat and mouse liver. Both enzyme preparations were specific for L-alanine and glyoxylate. On the basis of physical properties and substrate specificity, it was concluded that isoenzyme 2 is a separate enzyme. Some other properties of isoenzymes 1 and 2 are described.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYD J. W. The intracellular distribution, latency and electrophoretic mobility of L-glutamate-oxaloacetate transaminase from rat liver. Biochem J. 1961 Nov;81:434–441. doi: 10.1042/bj0810434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C. B., Civen M. Control of rat liver aromatic amino acid transaminases by glucagon and insulin. Endocrinology. 1969 Feb;84(2):381–385. doi: 10.1210/endo-84-2-381. [DOI] [PubMed] [Google Scholar]
- Cheung G. P., Cotropia J. P., Sallach H. J. The effects of dietary protein on the hepatic enzymes of serine metabolism in the rabbit. Arch Biochem Biophys. 1969 Feb;129(2):672–682. doi: 10.1016/0003-9861(69)90227-6. [DOI] [PubMed] [Google Scholar]
- Civen M., Trimmer B. M., Brown C. B. The induction of hepatic tyrosine alpha-ketoglutarate and phenylalanine pyruvate transaminases by glucagon. Life Sci. 1967 Jun 15;6(12):1331–1338. doi: 10.1016/0024-3205(67)90029-x. [DOI] [PubMed] [Google Scholar]
- Cooper A. J. Asparagine transaminase from rat liver. J Biol Chem. 1977 Mar 25;252(6):2032–2038. [PubMed] [Google Scholar]
- Cooper J. L., Meister A. Isolation and properties of highly purified glutamine transaminase. Biochemistry. 1972 Feb 29;11(5):661–671. doi: 10.1021/bi00755a001. [DOI] [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller R. W., Baker J. C., Snoddy H. D. Elevation of hepatic phenylalanine: pyruvate aminotransferase by dibutyryl cyclic AMP in rats. Biochem Med. 1974 Mar;9(3):301–308. doi: 10.1016/0006-2944(74)90064-7. [DOI] [PubMed] [Google Scholar]
- Fuller R. W., Snoddy H. D., Wolen R. L., Coburn S. P., Sirlin E. M. Effect of glucagon and p-chlorophenylalanine on hepatic enzymes that metabolize phenylalanine. Adv Enzyme Regul. 1972;10:153–167. doi: 10.1016/0065-2571(72)90012-x. [DOI] [PubMed] [Google Scholar]
- Hancock I. C., Baddiley J. Biosynthesis of the wall teichoic acid in Bacillus licheniformis. Biochem J. 1972 Mar;127(1):27–37. doi: 10.1042/bj1270027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh B., Tolbert N. E. Glyoxylate aminotransferase in peroxisomes from rat liver and kidney. J Biol Chem. 1976 Jul 25;251(14):4408–4415. [PubMed] [Google Scholar]
- Ichihara A., Koyama E. Transaminase of branched chain amino acids. I. Branched chain amino acids-alpha-ketoglutarate transaminase. J Biochem. 1966 Feb;59(2):160–169. doi: 10.1093/oxfordjournals.jbchem.a128277. [DOI] [PubMed] [Google Scholar]
- Kupchik H. Z., Knox W. E. Assays of glutamine and its aminotransferase with the enol-borate of phenylpyruvate. Arch Biochem Biophys. 1970 Jan;136(1):178–186. doi: 10.1016/0003-9861(70)90339-5. [DOI] [PubMed] [Google Scholar]
- LIN E. C., PITT B. M., CIVEN M., KNOX W. E. The assay of aromatic amino acid transaminations and keto acid oxidation by the enol borate-tautomerase method. J Biol Chem. 1958 Sep;233(3):668–673. [PubMed] [Google Scholar]
- Morris M. L., Lee S. C., Harper A. E. A comparison of the responses of mitochondrial and cytosol histidine-pyruvate aminotransferases to nutritional and hormonal treatments. J Biol Chem. 1973 Feb 25;248(4):1459–1465. [PubMed] [Google Scholar]
- Nakatani Y., Fujioka M., Higashino K. Alpha-aminoadipate aminotransferase of rat liver mitochondria. Biochim Biophys Acta. 1970 Feb 11;198(2):219–228. doi: 10.1016/0005-2744(70)90054-9. [DOI] [PubMed] [Google Scholar]
- Noguchi T., Minatogawa Y., Okuno E., Kido R. Organ distribution of rat histidine-pyruvate aminotransferase isoenzymes. Biochem J. 1976 Sep 1;157(3):635–641. doi: 10.1042/bj1570635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T., Okuno E., Kido R. Identity of isoenzyme 1 of histidine-pyruvate aminotransferase with serine-pyruvate aminotransferase. Biochem J. 1976 Dec 1;159(3):607–613. doi: 10.1042/bj1590607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T., Okuno E., Kido R. Identity of rat kidney histidine-pyruvate aminotransferase with glutamine-oxo acid aminotransferase. Biochem J. 1977 Jan 1;161(1):177–179. doi: 10.1042/bj1610177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T., Okuno E., Minatogawa Y., Kido R. Purification, characterization and identification of rat liver histidine-pyruvate aminotransferase isoenzymes. Biochem J. 1976 Apr 1;155(1):107–115. doi: 10.1042/bj1550107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T., Takada Y., Kido R. Characteristics of hepatic serine-pyruvate aminotransferase in different mammalian species. Biochem J. 1977 Mar 1;161(3):609–614. doi: 10.1042/bj1610609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowsell E. V., Snell K., Carnie J. A., Al-Tai A. H. Liver-L-alanine-glyoxylate and L-serine-pyruvate aminotransferase activities: an apparent association with gluconeogenesis. Biochem J. 1969 Dec;115(5):1071–1073. doi: 10.1042/bj1151071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowsell E. V., Snell K., Carnie J. A., Rowsell K. V. The subcellular distribution of rat liver L-alanine-glyoxylate aminotransferase in relation to a pathway for glucose formation involving glyoxylate. Biochem J. 1972 Mar;127(1):155–165. doi: 10.1042/bj1270155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPOLTER H., BALDRIDGE R. C. MULTIPLE FORMS OF HISTIDINE-PYRUVATE TRANSAMINASE IN RAT LIVER. Biochim Biophys Acta. 1964 Aug 19;90:287–290. doi: 10.1016/0304-4165(64)90191-6. [DOI] [PubMed] [Google Scholar]
- SWICK R. W., BARNSTEIN P. L., STANGE J. L. THE METABOLISM OF MITOCHONDRIAL PROTEINS. I. DISTRIBUTION AND CHARACTERIZATION OF THE ISOZYMES OF ALANINE AMINOTRANSFERASE IN RAT LIVER. J Biol Chem. 1965 Aug;240:3334–3340. [PubMed] [Google Scholar]
- Sallach H. J., Sanborn T. A., Bruin W. J. Dietary and hormonal regulation of hepatic biosynthetic and catabolic enzymes of serine metabolism in rats. Endocrinology. 1972 Oct;91(4):1054–1063. doi: 10.1210/endo-91-4-1054. [DOI] [PubMed] [Google Scholar]
- Snell K. Mitochondrial-cytosolic interrelationships involved in gluconeogenesis from serine in rat liver. FEBS Lett. 1975 Jul 15;55(1):202–205. doi: 10.1016/0014-5793(75)80992-6. [DOI] [PubMed] [Google Scholar]
- Snell K. The regulation of rat liver L-alanine-glyoxylate aminotransferase by glucagon in vivo. Biochem J. 1971 Jul;123(4):657–659. doi: 10.1042/bj1230657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell K., Walker D. G. Regulation of hepatic L-serine dehydratase and L-serine-pyruvate aminotransferase in the developing neonatal rat. Biochem J. 1974 Dec;144(3):519–531. doi: 10.1042/bj1440519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell K., Walker D. G. The adaptive behaviour of isoenzyme forms of rat liver alanine aminotransferases during development. Biochem J. 1972 Jun;128(2):403–413. doi: 10.1042/bj1280403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. S., Richardson K. E. Isolation and characterization of an L-alanine: glyoxylate aminotransferase from human liver. J Biol Chem. 1967 Aug 25;242(16):3614–3619. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]


