Abstract
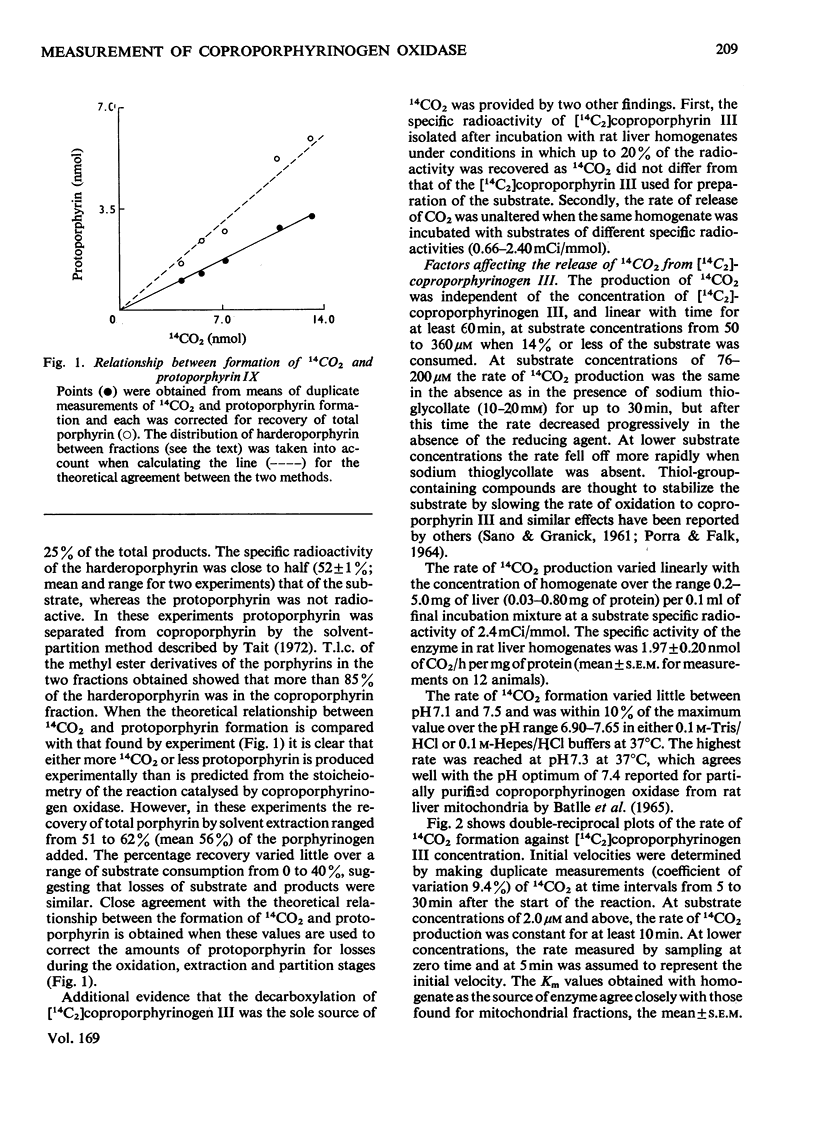
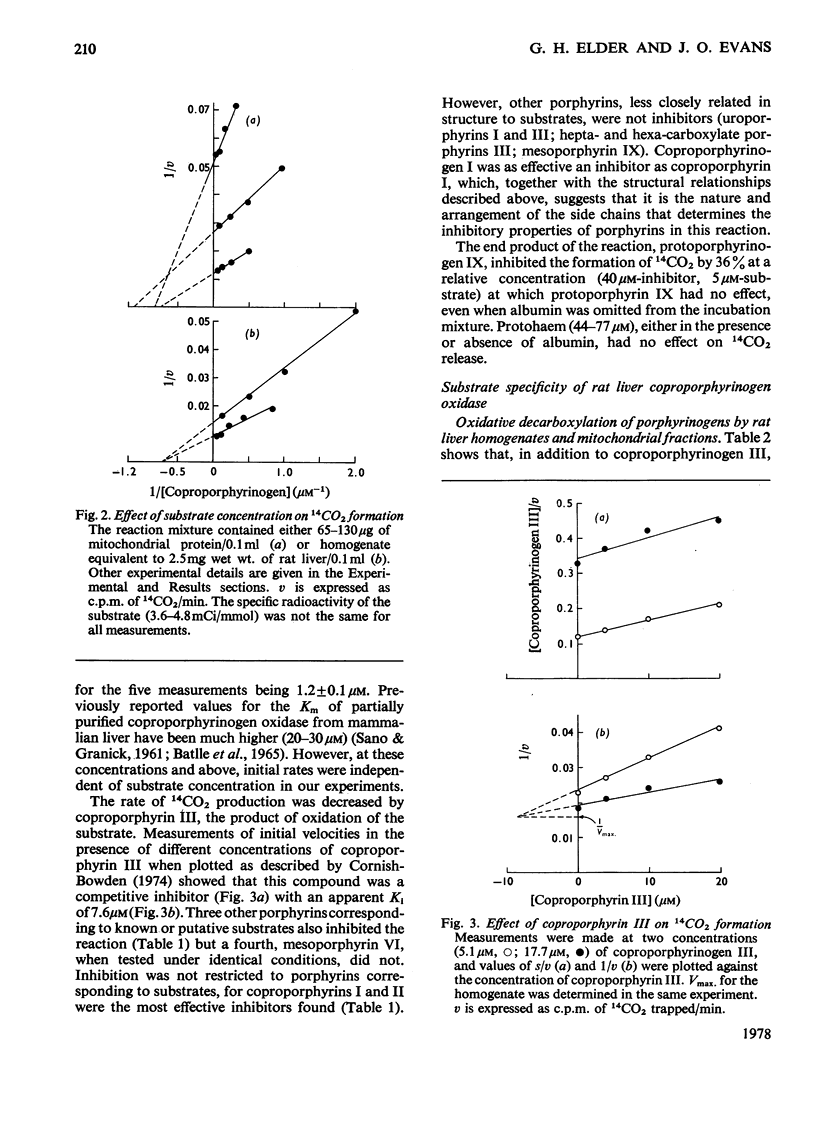
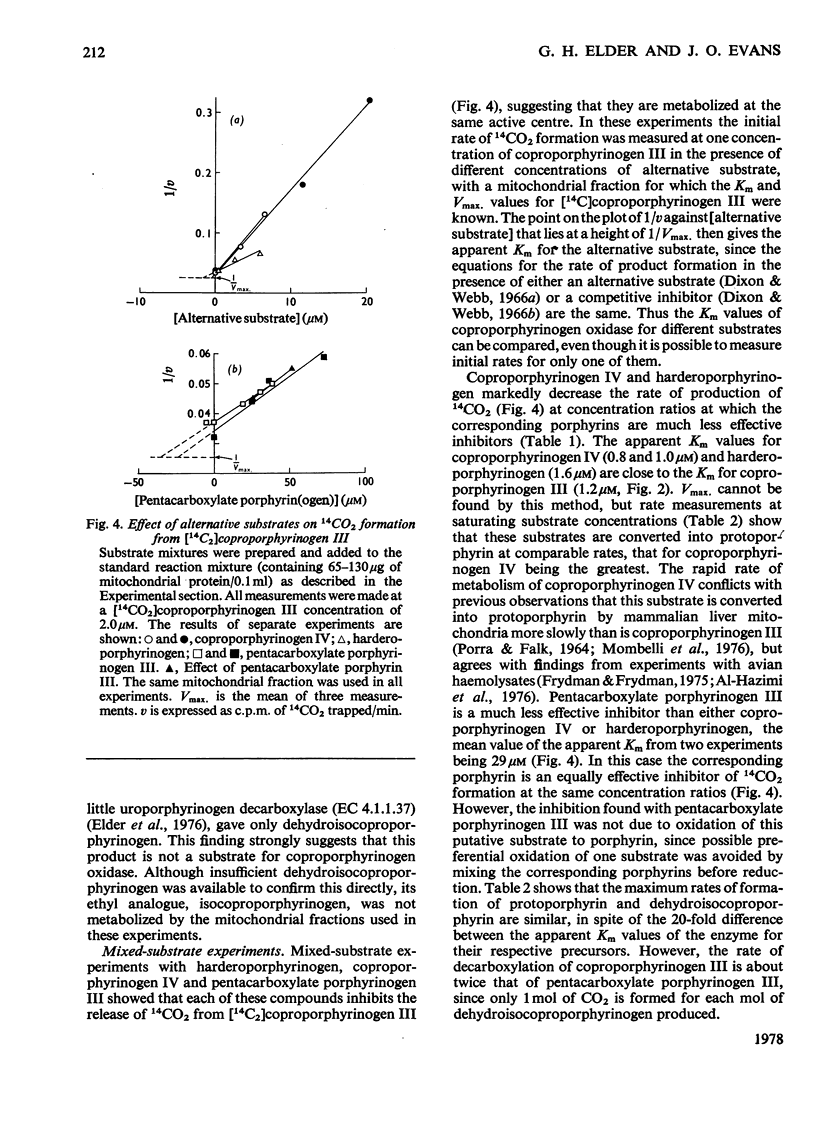
[14C2]Coproporphyrin III, 14C-labelled in the carboxyl carbon atoms of the 2- and 4-propionate substituents, was prepared by stepwise modification of the vinyl groups of protoporphyrin IX. The corresponding porphyrinogen was used as substrate in a specific sensitive assay for coproporphyrinogen oxidase (EC 1.3.3.3) in which the rate of production of 14CO2 is measured. With this method, the Km of the enzyme from rat liver for coproporphyrinogen III is 1.2 micron. Coproporphyrin III is a competitive inhibitor of the enzyme (Ki 7.6 micron). Apparent Km values for other substrates were measured by a mixed-substrate method: that for coproporphyrinogen IV is 0.9 micron and that for harderoporphyrinogen 1.6 micron. Rat liver mitochondria convert pentacarboxylate porphyrinogen III into dehydroisocoproporphyrinogen at a rate similar to that for the formation of protoporphyrinogen IX from coproporphyrinogen III. Mixed-substrate experiments indicate that this reaction is catalysed by coproporphyrinogen oxidase and that the Km for this substrate is 29 micron. It is suggested that the ratio of the concentration of pentacarboxylate porphyrinogen III to coproporphyrinogen III in the hepatocyte determines the relative rates of formation of dehydroisocoproporphyrinogen and protoporphyrinogen IX.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cornish-Bowden A. A simple graphical method for determining the inhibition constants of mixed, uncompetitive and non-competitive inhibitors. Biochem J. 1974 Jan;137(1):143–144. doi: 10.1042/bj1370143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis F., Abbritti G., Gibbs A. H. Decreased liver activity of porphyrin-metal chelatase in hepatic porphyria caused by 3,5-diethoxycarbonyl-1,4-dihydrocollidine. Studies in rats and mice. Biochem J. 1973 Jul;134(3):717–727. doi: 10.1042/bj1340717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDMONDSON P. R., SCHWARTZ S. Studies of the uroporphyrins. III. An improved method for the decarboxylation of uroporphyrin. J Biol Chem. 1953 Dec;205(2):605–609. [PubMed] [Google Scholar]
- Elder G. H. Differentiation of porphyria cutanea tarda symptomatica from other types of porphyria by measurement of isocoproporphyrin in faeces. J Clin Pathol. 1975 Aug;28(8):601–607. doi: 10.1136/jcp.28.8.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder G. H., Evans J. O., Jackson J. R., Jackson A. H. Factors determining the sequence of oxidative decarboxylation of the 2- and 4-propionate substituents of coproporphyrinogen III by coproporphyrinogen oxidase in rat liver. Biochem J. 1978 Jan 1;169(1):215–223. doi: 10.1042/bj1690215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder G. H., Evans J. O., Matlin S. A. The effect of the porphyrogenic compound, hexachlorobenzene, on the activity of hepatic uroporphyrinogen decarboxylase in the rat. Clin Sci Mol Med. 1976 Jul;51(1):71–80. doi: 10.1042/cs0510071. [DOI] [PubMed] [Google Scholar]
- Elder G. H. Identification of a group of tetracarboxylate porphyrins, containing one acetate and three propionate -substituents, in faeces from patients with symptomatic cutaneous hepatic porphyria and from rats with porphyria due to hexachlorobenzene. Biochem J. 1972 Feb;126(4):877–891. doi: 10.1042/bj1260877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder G. H. Porphyrin metabolism in porphyria cutanea tarda. Semin Hematol. 1977 Apr;14(2):227–242. [PubMed] [Google Scholar]
- Elder G. H. The metabolism of porphyrins of the isocoproporphyrin series. Enzyme. 1974;17(1):61–68. doi: 10.1159/000459308. [DOI] [PubMed] [Google Scholar]
- Frydman R. B., Frydman B. Formation of uroporphyrinogen IV during the chemical dimerization of 2-aminomethyl-3',3'-carboxymethyl-4,4' (beta-carboxyethyl) dipyrrylmethane. FEBS Lett. 1975 Apr 1;52(2):317–322. doi: 10.1016/0014-5793(75)80834-9. [DOI] [PubMed] [Google Scholar]
- GRANICK S., LEVERE R. D. HEME SYNTHESIS IN ERYTHROID CELLS. Prog Hematol. 1964;4:1–47. [PubMed] [Google Scholar]
- Hsu W. P., Miller G. W. Coproporphyrinogenase in tobacco (Nicotiana tabacum L.). Biochem J. 1970 Apr;117(2):215–220. doi: 10.1042/bj1170215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A. H., Sancovich H. A., Ferramola A. M., Evans N., Games D. E., Matlin S. A., Elder G. H., Smith S. G. Macrocyclic intermediates in the biosynthesis of porphyrins. Philos Trans R Soc Lond B Biol Sci. 1976 Feb 5;273(924):191–206. doi: 10.1098/rstb.1976.0009. [DOI] [PubMed] [Google Scholar]
- Kennedy G. Y., Jackson A. H., Kenner G. W., Suckling C. J. Isolation, structure and synthesis of a tricarboxylic porphyrin from the harderian glands of the rat. FEBS Lett. 1970 Jan 15;6(1):9–12. doi: 10.1016/0014-5793(70)80027-8. [DOI] [PubMed] [Google Scholar]
- Kushner J. P., Barbuto A. J., Lee G. R. An inherited enzymatic defect in porphyria cutanea tarda: decreased uroporphyrinogen decarboxylase activity. J Clin Invest. 1976 Nov;58(5):1089–1097. doi: 10.1172/JCI108560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Porra R. J., Falk J. E. The enzymic conversion of coproporphyrinogen 3 into protoporphyrin 9. Biochem J. 1964 Jan;90(1):69–75. doi: 10.1042/bj0900069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulson R., Polglase W. J. Aerobic and anaerobic coproporphyrinogenase activities in extracts from Saccharomyces cerevisiae. J Biol Chem. 1974 Oct 25;249(20):6367–6371. [PubMed] [Google Scholar]
- SANO S., GRANICK S. Mitochondrial coproporphyrinogen oxidase and protoporphyrin formation. J Biol Chem. 1961 Apr;236:1173–1180. [PubMed] [Google Scholar]
- Sano S. 2,4-Bis-(beta-hydroxypropionic acid) deuteroporphyrinogen IX, a possible intermediate between coproporphyrinogen 3 and protoporphyrin IX. J Biol Chem. 1966 Nov 25;241(22):5276–5283. [PubMed] [Google Scholar]
- Stoll M. S., Elder G. H., Games D. E., O'Hanlon P., Millington D. S., Jackson A. H. Isocoproporphyrin: nuclear-magnetic-resonance-and mass-spectral methods for the determination of porphyrin structure. Biochem J. 1973 Feb;131(2):429–432. doi: 10.1042/bj1310429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait G. H. Coproporphyrinogenase activities in extracts of Rhodopseudomonas spheroides and Chromatium strain D. Biochem J. 1972 Aug;128(5):1159–1169. doi: 10.1042/bj1281159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WITH T. K. Uroporphyrin from turacin: a simplified method. Nature. 1957 Apr 20;179(4564):824–824. doi: 10.1038/179824a0. [DOI] [PubMed] [Google Scholar]
- Watson C. J., Cardinal R. A., Bossenmaier I., Petryka Z. J. Porphyria variegata and porphyria cutanea tarda in siblings: chemical and genetic aspects. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5126–5129. doi: 10.1073/pnas.72.12.5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Batlle A. M., Benson A., Rimington C. Purification and properties of coproporphyrinogenase. Biochem J. 1965 Dec;97(3):731–740. doi: 10.1042/bj0970731. [DOI] [PMC free article] [PubMed] [Google Scholar]


