Abstract
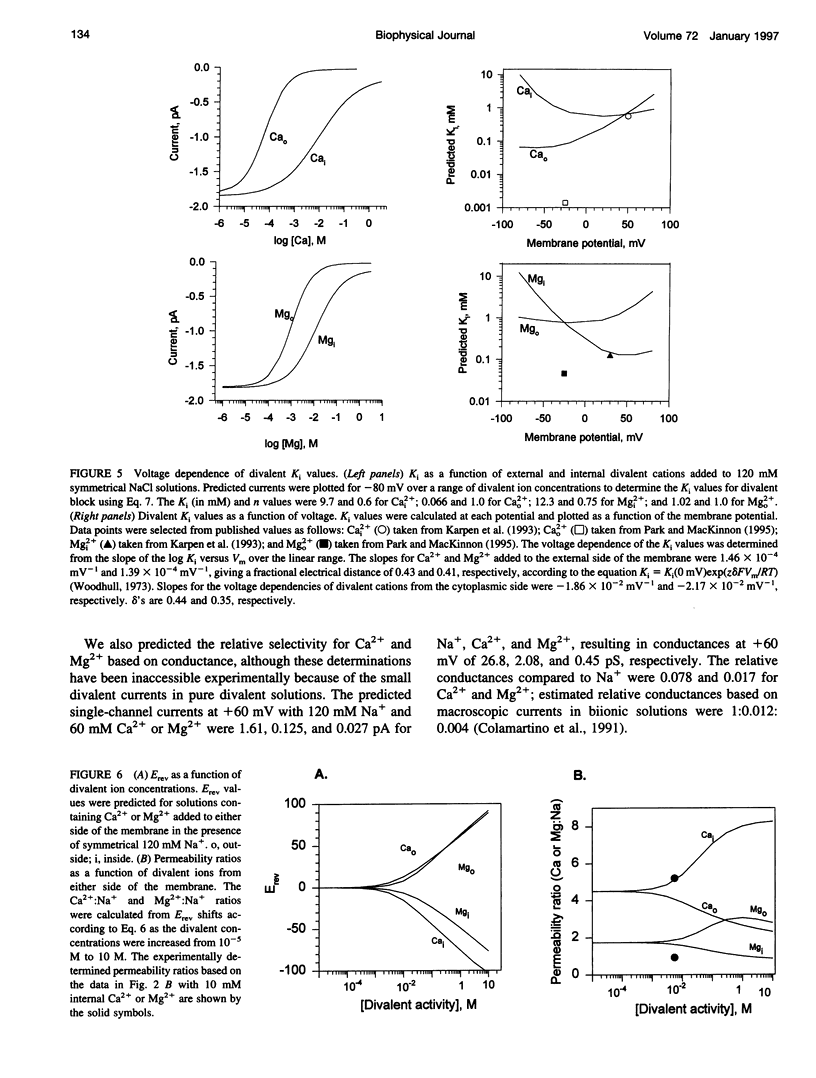
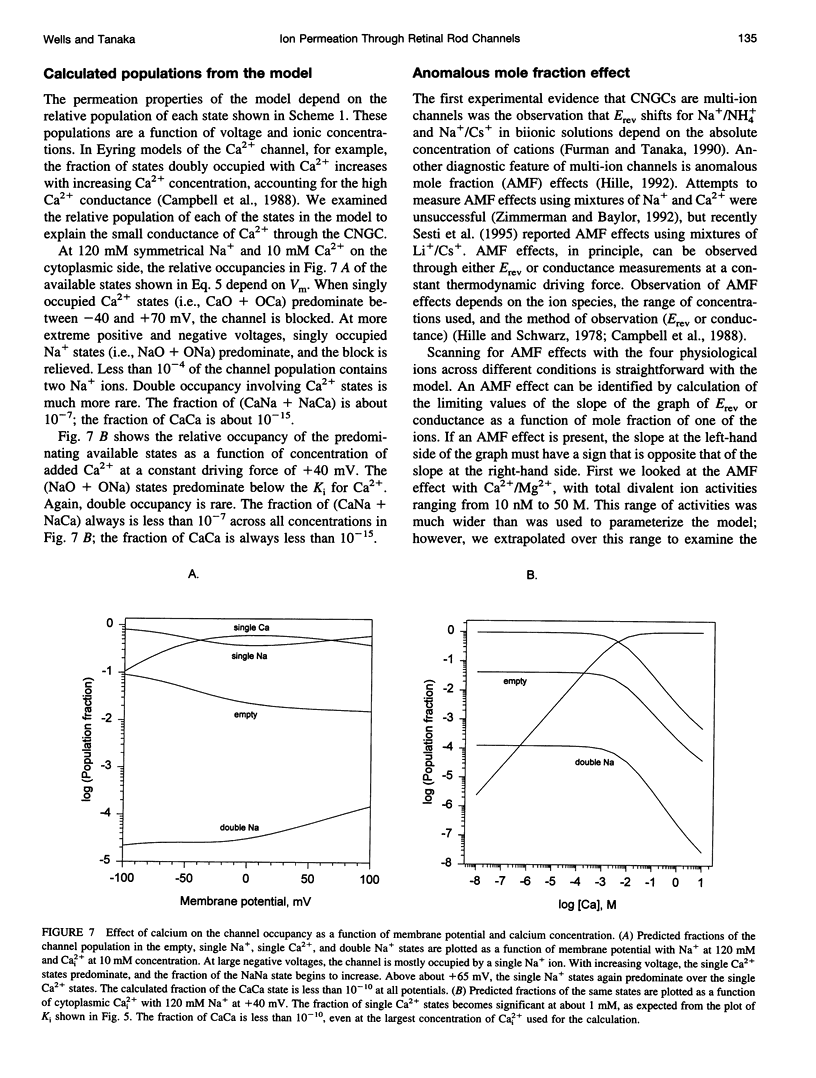
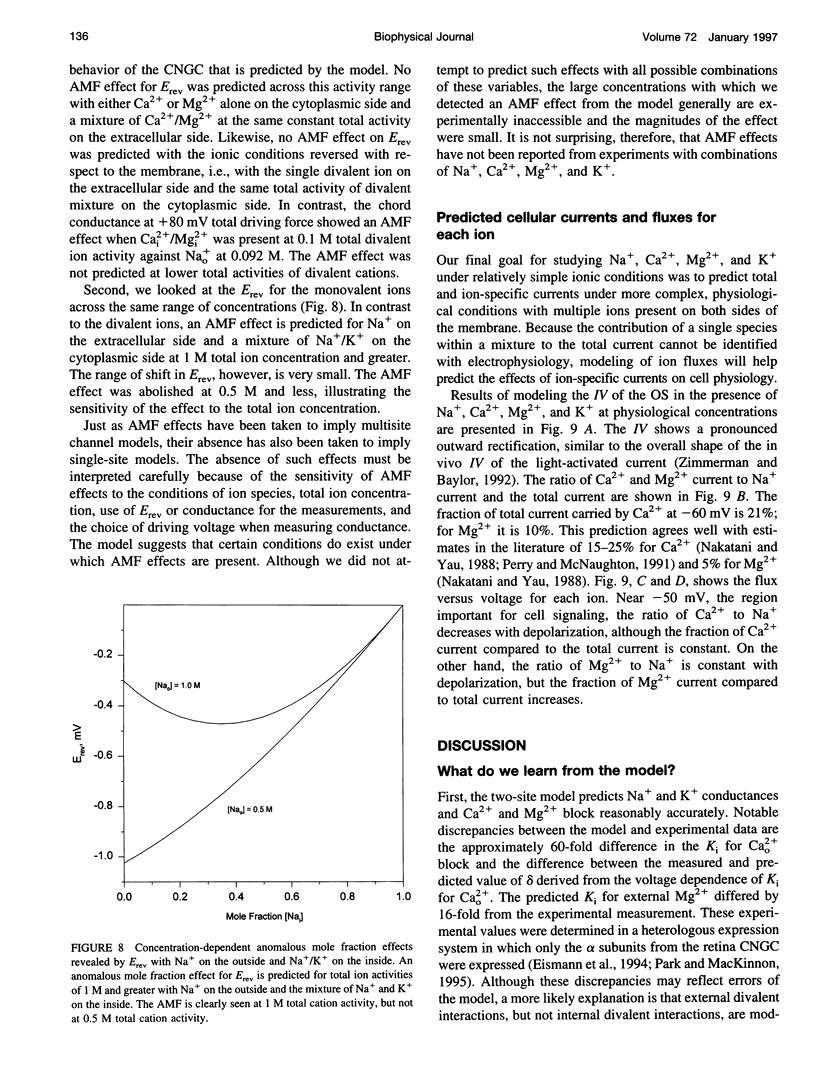
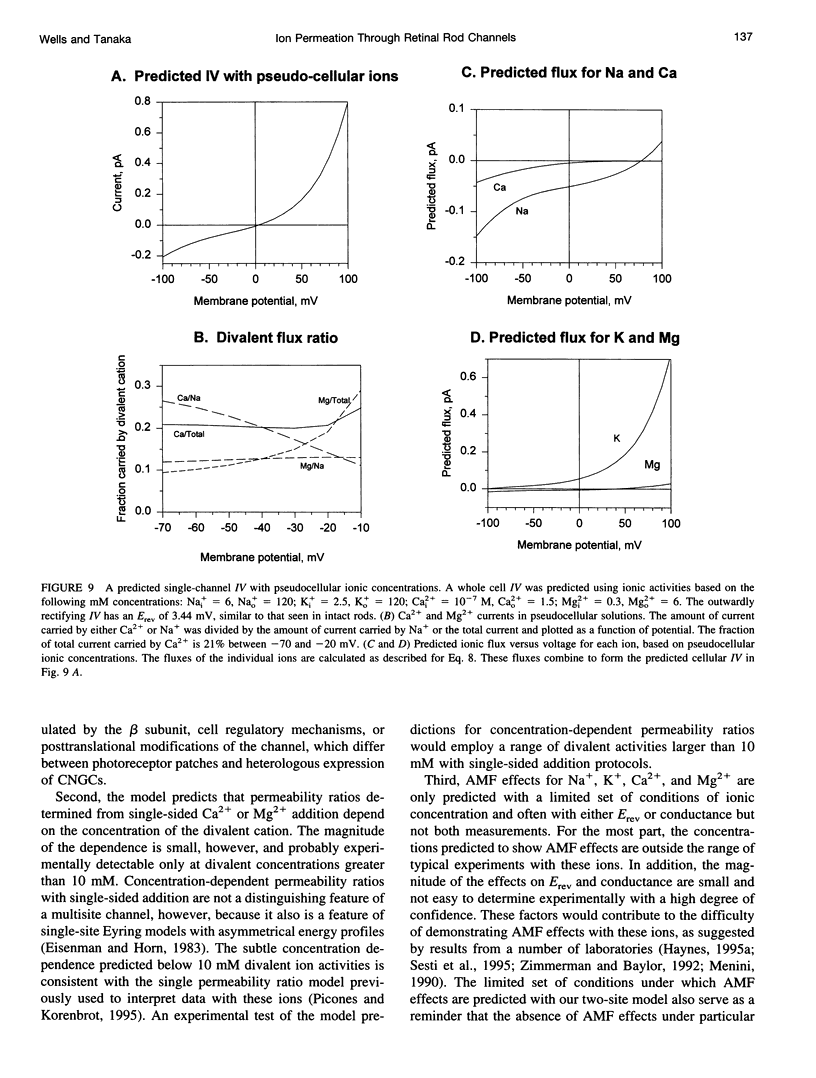
We developed a two-site, Eyring rate theory model of ionic permeation for cyclic nucleotide-gated channels (CNGCs). The parameters of the model were optimized by simultaneously fitting current-voltage (IV) data sets from excised photoreceptor patches in electrolyte solutions containing one or more of the following ions: Na+, Ca2+, Mg2+, and K+. The model accounted well for 1) the shape of the IV relations; 2) the binding affinity for Na+; 3) reversal potential values with single-sided additions of Ca2+ or Mg2+ and biionic KCl; and 4) the K1 and voltage dependence for divalent block from the cytoplasmic side of the channel. The differences between the predicted K1's for extracellular block by Ca2+ and Mg2+ and the values obtained from heterologous expression of only the alpha-subunit of the channel suggest that the beta-subunit or a cell-specific factor affects the interaction of divalent cations at the external but not the internal face of the channel. The model predicts concentration-dependent permeability ratios with single-sided addition of Ca2+ and Mg2+ and anomalous mole fraction effects under a limited set of conditions for both monovalent and divalent cations. Ca2+ and Mg2+ are predicted to carry 21% and 10%, respectively, of the total current in the retinal rod cell at -60 mV.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez O., Villarroel A., Eisenman G. Calculation of ion currents from energy profiles and energy profiles from ion currents in multibarrier, multisite, multioccupancy channel model. Methods Enzymol. 1992;207:816–854. doi: 10.1016/0076-6879(92)07058-v. [DOI] [PubMed] [Google Scholar]
- Begenisich T. B., Cahalan M. D. Sodium channel permeation in squid axons. I: Reversal potential experiments. J Physiol. 1980 Oct;307:217–242. doi: 10.1113/jphysiol.1980.sp013432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley J., Li J., Davidson N., Lester H. A., Zinn K. Heteromeric olfactory cyclic nucleotide-gated channels: a subunit that confers increased sensitivity to cAMP. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):8890–8894. doi: 10.1073/pnas.91.19.8890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler J. N. The thermodynamic activity of calcium ion in sodium chloride-calcium chloride electrolytes. Biophys J. 1968 Dec;8(12):1426–1433. doi: 10.1016/S0006-3495(68)86564-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell D. L., Rasmusson R. L., Strauss H. C. Theoretical study of the voltage and concentration dependence of the anomalous mole fraction effect in single calcium channels. New insights into the characterization of multi-ion channels. Biophys J. 1988 Nov;54(5):945–954. doi: 10.1016/S0006-3495(88)83030-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colamartino G., Menini A., Torre V. Blockage and permeation of divalent cations through the cyclic GMP-activated channel from tiger salamander retinal rods. J Physiol. 1991;440:189–206. doi: 10.1113/jphysiol.1991.sp018703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper K. E., Gates P. Y., Eisenberg R. S. Diffusion theory and discrete rate constants in ion permeation. J Membr Biol. 1988 Dec;106(2):95–105. doi: 10.1007/BF01871391. [DOI] [PubMed] [Google Scholar]
- Eisenman G., Horn R. Ionic selectivity revisited: the role of kinetic and equilibrium processes in ion permeation through channels. J Membr Biol. 1983;76(3):197–225. doi: 10.1007/BF01870364. [DOI] [PubMed] [Google Scholar]
- Eismann E., Müller F., Heinemann S. H., Kaupp U. B. A single negative charge within the pore region of a cGMP-gated channel controls rectification, Ca2+ blockage, and ionic selectivity. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):1109–1113. doi: 10.1073/pnas.91.3.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frings S., Seifert R., Godde M., Kaupp U. B. Profoundly different calcium permeation and blockage determine the specific function of distinct cyclic nucleotide-gated channels. Neuron. 1995 Jul;15(1):169–179. doi: 10.1016/0896-6273(95)90074-8. [DOI] [PubMed] [Google Scholar]
- Furman R. E., Tanaka J. C. Monovalent selectivity of the cyclic guanosine monophosphate-activated ion channel. J Gen Physiol. 1990 Jul;96(1):57–82. doi: 10.1085/jgp.96.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulding E. H., Tibbs G. R., Liu D., Siegelbaum S. A. Role of H5 domain in determining pore diameter and ion permeation through cyclic nucleotide-gated channels. Nature. 1993 Jul 1;364(6432):61–64. doi: 10.1038/364061a0. [DOI] [PubMed] [Google Scholar]
- Haynes L. W., Kay A. R., Yau K. W. Single cyclic GMP-activated channel activity in excised patches of rod outer segment membrane. Nature. 1986 May 1;321(6065):66–70. doi: 10.1038/321066a0. [DOI] [PubMed] [Google Scholar]
- Haynes L. W. Permeation and block by internal and external divalent cations of the catfish cone photoreceptor cGMP-gated channel. J Gen Physiol. 1995 Sep;106(3):507–523. doi: 10.1085/jgp.106.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes L. W. Permeation of internal and external monovalent cations through the catfish cone photoreceptor cGMP-gated channel. J Gen Physiol. 1995 Sep;106(3):485–505. doi: 10.1085/jgp.106.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ionic selectivity, saturation, and block in sodium channels. A four-barrier model. J Gen Physiol. 1975 Nov;66(5):535–560. doi: 10.1085/jgp.66.5.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B., Schwarz W. Potassium channels as multi-ion single-file pores. J Gen Physiol. 1978 Oct;72(4):409–442. doi: 10.1085/jgp.72.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ildefonse M., Bennett N. Single-channel study of the cGMP-dependent conductance of retinal rods from incorporation of native vesicles into planar lipid bilayers. J Membr Biol. 1991 Aug;123(2):133–147. doi: 10.1007/BF01998084. [DOI] [PubMed] [Google Scholar]
- Karpen J. W., Brown R. L., Stryer L., Baylor D. A. Interactions between divalent cations and the gating machinery of cyclic GMP-activated channels in salamander retinal rods. J Gen Physiol. 1993 Jan;101(1):1–25. doi: 10.1085/jgp.101.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaupp U. B., Koch K. W. Role of cGMP and Ca2+ in vertebrate photoreceptor excitation and adaptation. Annu Rev Physiol. 1992;54:153–175. doi: 10.1146/annurev.ph.54.030192.001101. [DOI] [PubMed] [Google Scholar]
- Kaupp U. B. The cyclic nucleotide-gated channels of vertebrate photoreceptors and olfactory epithelium. Trends Neurosci. 1991 Apr;14(4):150–157. doi: 10.1016/0166-2236(91)90087-b. [DOI] [PubMed] [Google Scholar]
- Kienker P. K., DeGrado W. F., Lear J. D. A helical-dipole model describes the single-channel current rectification of an uncharged peptide ion channel. Proc Natl Acad Sci U S A. 1994 May 24;91(11):4859–4863. doi: 10.1073/pnas.91.11.4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienker P. K., Lear J. D. Charge selectivity of the designed uncharged peptide ion channel Ac-(LSSLLSL)3-CONH2. Biophys J. 1995 Apr;68(4):1347–1358. doi: 10.1016/S0006-3495(95)80307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt D. G. Electrostatic calculations for an ion channel. I. Energy and potential profiles and interactions between ions. Biophys J. 1978 May;22(2):209–219. doi: 10.1016/S0006-3495(78)85485-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis C. A. Ion-concentration dependence of the reversal potential and the single channel conductance of ion channels at the frog neuromuscular junction. J Physiol. 1979 Jan;286:417–445. doi: 10.1113/jphysiol.1979.sp012629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liman E. R., Buck L. B. A second subunit of the olfactory cyclic nucleotide-gated channel confers high sensitivity to cAMP. Neuron. 1994 Sep;13(3):611–621. doi: 10.1016/0896-6273(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Matthews G. Comparison of the light-sensitive and cyclic GMP-sensitive conductances of the rod photoreceptor: noise characteristics. J Neurosci. 1986 Sep;6(9):2521–2526. doi: 10.1523/JNEUROSCI.06-09-02521.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menini A. Currents carried by monovalent cations through cyclic GMP-activated channels in excised patches from salamander rods. J Physiol. 1990 May;424:167–185. doi: 10.1113/jphysiol.1990.sp018061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani K., Yau K. W. Calcium and magnesium fluxes across the plasma membrane of the toad rod outer segment. J Physiol. 1988 Jan;395:695–729. doi: 10.1113/jphysiol.1988.sp016942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizzari M., Sesti F., Giraudo M. T., Virginio C., Cattaneo A., Torre V. Single-channel properties of cloned cGMP-activated channels from retinal rods. Proc Biol Sci. 1993 Oct 22;254(1339):69–74. doi: 10.1098/rspb.1993.0128. [DOI] [PubMed] [Google Scholar]
- Park C. S., MacKinnon R. Divalent cation selectivity in a cyclic nucleotide-gated ion channel. Biochemistry. 1995 Oct 17;34(41):13328–13333. doi: 10.1021/bi00041a008. [DOI] [PubMed] [Google Scholar]
- Perry R. J., McNaughton P. A. Response properties of cones from the retina of the tiger salamander. J Physiol. 1991 Feb;433:561–587. doi: 10.1113/jphysiol.1991.sp018444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picones A., Korenbrot J. I. Permeability and interaction of Ca2+ with cGMP-gated ion channels differ in retinal rod and cone photoreceptors. Biophys J. 1995 Jul;69(1):120–127. doi: 10.1016/S0006-3495(95)79881-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picones A., Korenbrot J. I. Permeation and interaction of monovalent cations with the cGMP-gated channel of cone photoreceptors. J Gen Physiol. 1992 Oct;100(4):647–673. doi: 10.1085/jgp.100.4.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Cornejo P., Begenisich T. The multi-ion nature of the pore in Shaker K+ channels. Biophys J. 1994 Jun;66(6):1929–1938. doi: 10.1016/S0006-3495(94)80986-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root M. J., MacKinnon R. Identification of an external divalent cation-binding site in the pore of a cGMP-activated channel. Neuron. 1993 Sep;11(3):459–466. doi: 10.1016/0896-6273(93)90150-p. [DOI] [PubMed] [Google Scholar]
- Sesti F., Eismann E., Kaupp U. B., Nizzari M., Torre V. The multi-ion nature of the cGMP-gated channel from vertebrate rods. J Physiol. 1995 Aug 15;487(1):17–36. doi: 10.1113/jphysiol.1995.sp020858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesti F., Nizzari M., Torre V. Effect of changing temperature on the ionic permeation through the cyclic GMP-gated channel from vertebrate photoreceptors. Biophys J. 1996 Jun;70(6):2616–2639. doi: 10.1016/S0006-3495(96)79832-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesti F., Straforini M., Lamb T. D., Torre V. Gating, selectivity and blockage of single channels activated by cyclic GMP in retinal rods of the tiger salamander. J Physiol. 1994 Jan 15;474(2):203–222. doi: 10.1113/jphysiol.1994.sp020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka J. C., Furman R. E. Divalent effects on cGMP-activated currents in excised patches from amphibian photoreceptors. J Membr Biol. 1993 Feb;131(3):245–256. doi: 10.1007/BF02260113. [DOI] [PubMed] [Google Scholar]
- Woodhull A. M. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973 Jun;61(6):687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau K. W., Baylor D. A. Cyclic GMP-activated conductance of retinal photoreceptor cells. Annu Rev Neurosci. 1989;12:289–327. doi: 10.1146/annurev.ne.12.030189.001445. [DOI] [PubMed] [Google Scholar]
- Yau K. W. Calcium and light adaptation in retinal photoreceptors. Curr Opin Neurobiol. 1991 Aug;1(2):252–257. doi: 10.1016/0959-4388(91)90086-m. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Light-induced reduction of cytoplasmic free calcium in retinal rod outer segment. Nature. 1985 Feb 14;313(6003):579–582. doi: 10.1038/313579a0. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Light-suppressible, cyclic GMP-sensitive conductance in the plasma membrane of a truncated rod outer segment. Nature. 1985 Sep 19;317(6034):252–255. doi: 10.1038/317252a0. [DOI] [PubMed] [Google Scholar]
- Zagotta W. N., Siegelbaum S. A. Structure and function of cyclic nucleotide-gated channels. Annu Rev Neurosci. 1996;19:235–263. doi: 10.1146/annurev.ne.19.030196.001315. [DOI] [PubMed] [Google Scholar]
- Zimmerman A. L., Baylor D. A. Cation interactions within the cyclic GMP-activated channel of retinal rods from the tiger salamander. J Physiol. 1992 Apr;449:759–783. doi: 10.1113/jphysiol.1992.sp019112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman A. L., Baylor D. A. Cyclic GMP-sensitive conductance of retinal rods consists of aqueous pores. Nature. 1986 May 1;321(6065):70–72. doi: 10.1038/321070a0. [DOI] [PubMed] [Google Scholar]


