Abstract
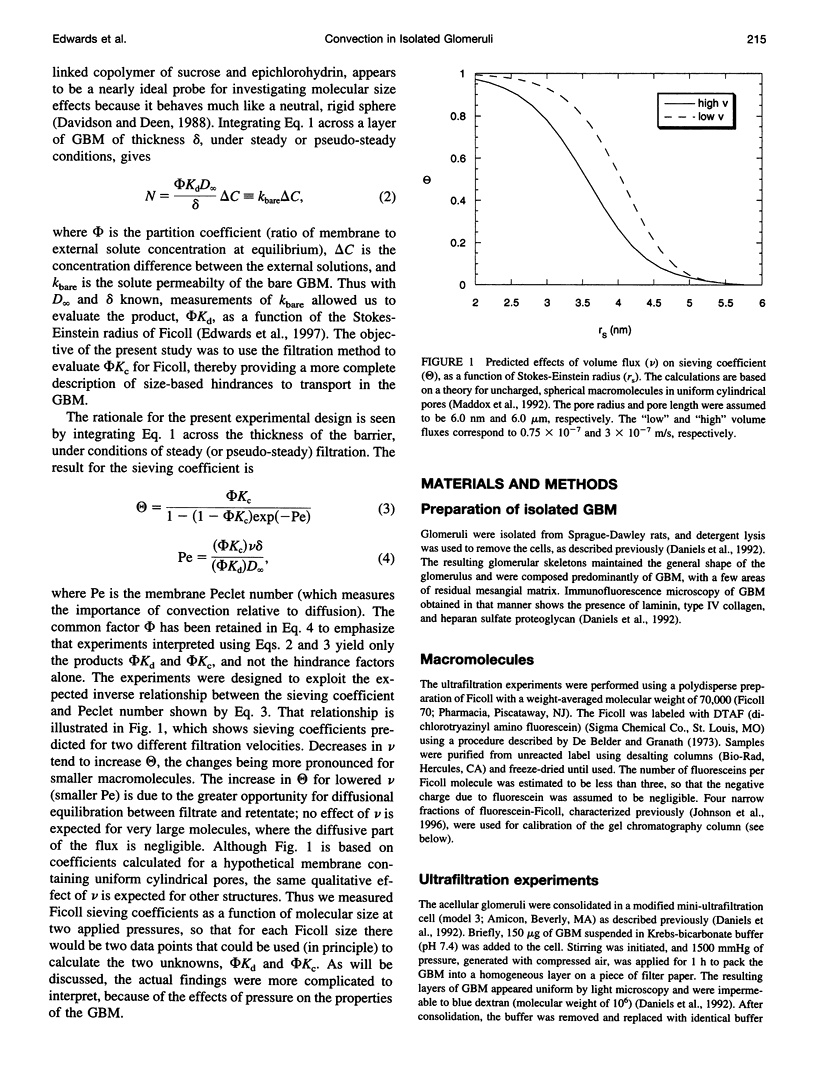
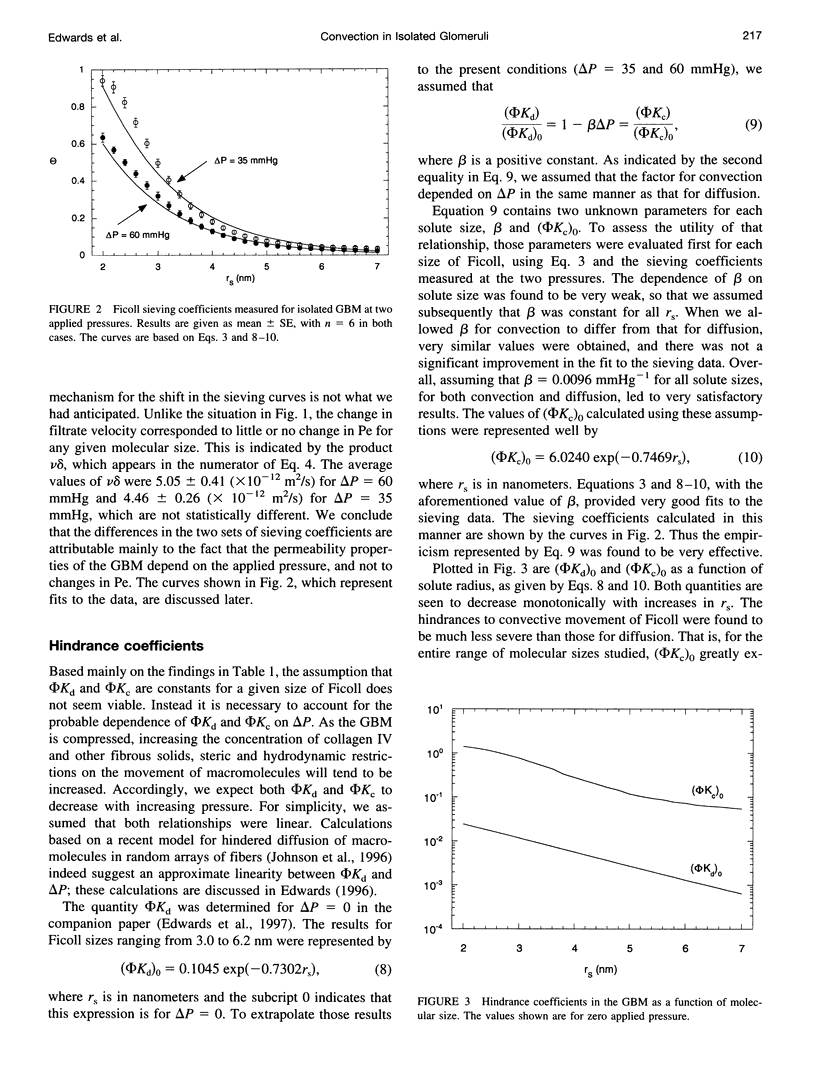
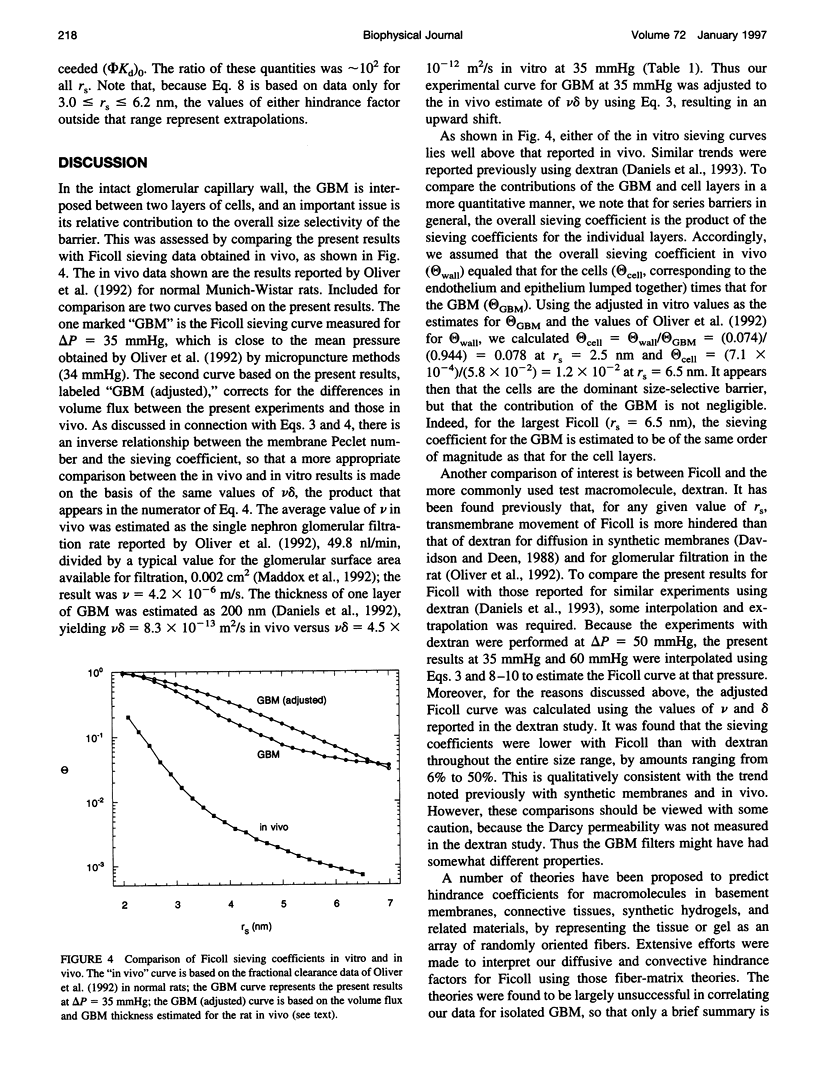
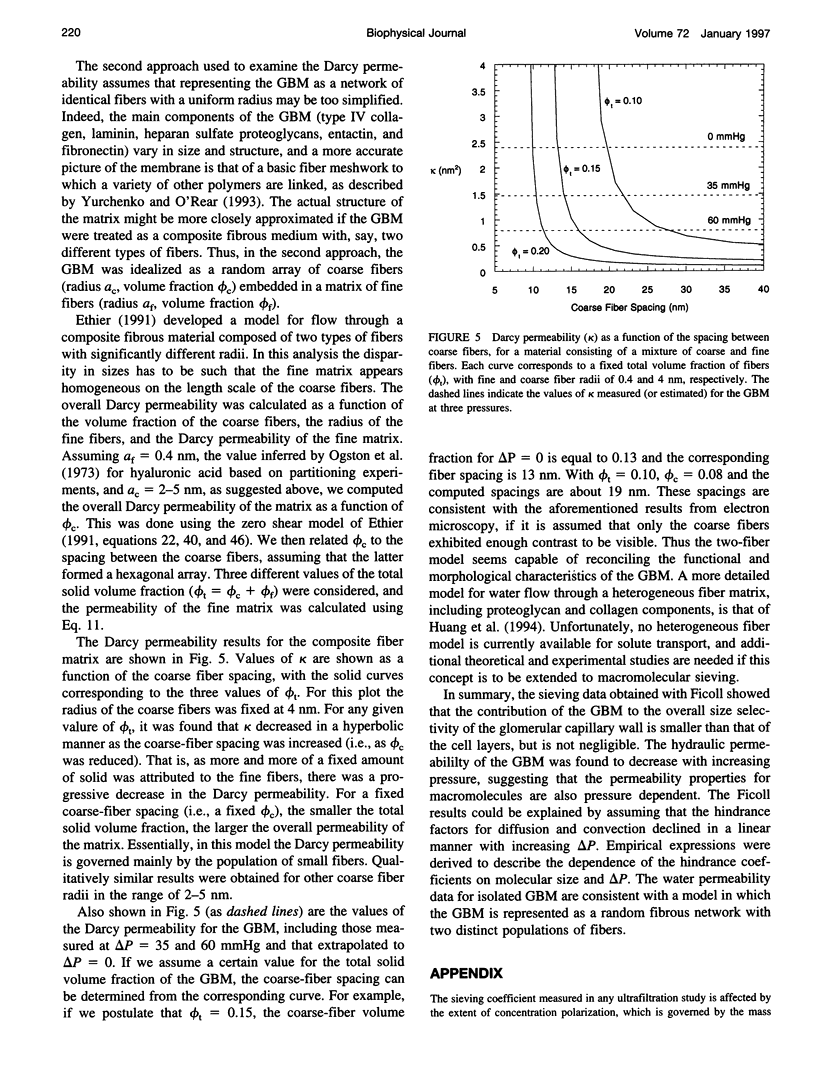
The filtration rates for water and a polydisperse mixture of Ficoll across films of isolated glomerular basement membrane (GBM) were measured to characterize convective transport across this part of the glomerular capillary wall. Glomeruli were isolated from rat kidneys and the cells were removed by detergent lysis, leaving a preparation containing almost pure GBM that could be consolidated into a layer at the base of a small ultrafiltration cell. A Ficoll mixture with Stokes-Einstein radii ranging from about 2.0 to 7.0 nm was labeled with fluorescein, providing a set of rigid, spherical test macromolecules with little molecular charge. Filtration experiments were performed at two physiologically relevant hydraulic pressure differences (delta P), 35 and 60 mmHg. The sieving coefficient (filtrate-to-retentate concentration ratio) for a given size of Ficoll tended to be larger at 35 than at 60 mmHg, the changes being greater for the smaller molecules. The Darcy permeability also varied inversely with pressure, averaging 1.48 +/- 0.10 nm2 at 35 mmHg and 0.82 +/- 0.07 nm2 at 60 mmHg. Both effects could be explained most simply by postulating that the intrinsic permeability properties of the GBM change in response to compression. The sieving data were consistent with linear declines in the hindrance factors for convection and diffusion with increasing pressure, and correlations were derived to relate those hindrance factors to molecular size and delta P. Comparisons with previous Ficoll sieving data for rats in vivo suggest that the GBM is less size-restrictive than the cell layers, but that its contribution to the overall size selectivity of the barrier is not negligible. Theoretical predictions of the Darcy permeability based on a model in which the GBM is a random fibrous network consisting of two populations of fibers were in excellent agreement with the present data and with ultrastructural observations in the literature.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Curry F. E., Michel C. C. A fiber matrix model of capillary permeability. Microvasc Res. 1980 Jul;20(1):96–99. doi: 10.1016/0026-2862(80)90024-2. [DOI] [PubMed] [Google Scholar]
- DOHLMAN C. H., BALAZS E. A. Chemical studies on Descemet's membrane of the bovine cornea. Arch Biochem Biophys. 1955 Aug;57(2):445–457. doi: 10.1016/0003-9861(55)90306-4. [DOI] [PubMed] [Google Scholar]
- Daniels B. S., Deen W. M., Mayer G., Meyer T., Hostetter T. H. Glomerular permeability barrier in the rat. Functional assessment by in vitro methods. J Clin Invest. 1993 Aug;92(2):929–936. doi: 10.1172/JCI116668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels B. S., Hauser E. B., Deen W. M., Hostetter T. H. Glomerular basement membrane: in vitro studies of water and protein permeability. Am J Physiol. 1992 Jun;262(6 Pt 2):F919–F926. doi: 10.1152/ajprenal.1992.262.6.F919. [DOI] [PubMed] [Google Scholar]
- Daniels B. S. Increased albumin permeability in vitro following alterations of glomerular charge is mediated by the cells of the filtration barrier. J Lab Clin Med. 1994 Aug;124(2):224–230. [PubMed] [Google Scholar]
- Huang Y., Rumschitzki D., Chien S., Weinbaum S. A fiber matrix model for the growth of macromolecular leakage spots in the arterial intima. J Biomech Eng. 1994 Nov;116(4):430–445. doi: 10.1115/1.2895794. [DOI] [PubMed] [Google Scholar]
- Johnson E. M., Berk D. A., Jain R. K., Deen W. M. Hindered diffusion in agarose gels: test of effective medium model. Biophys J. 1996 Feb;70(2):1017–1023. doi: 10.1016/S0006-3495(96)79645-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubosawa H., Kondo Y. Ultrastructural organization of the glomerular basement membrane as revealed by a deep-etch replica method. Cell Tissue Res. 1985;242(1):33–39. doi: 10.1007/BF00225560. [DOI] [PubMed] [Google Scholar]
- Laurie G. W., Leblond C. P., Inoue S., Martin G. R., Chung A. Fine structure of the glomerular basement membrane and immunolocalization of five basement membrane components to the lamina densa (basal lamina) and its extensions in both glomeruli and tubules of the rat kidney. Am J Anat. 1984 Apr;169(4):463–481. doi: 10.1002/aja.1001690408. [DOI] [PubMed] [Google Scholar]
- Oliver J. D., 3rd, Anderson S., Troy J. L., Brenner B. M., Deen W. H. Determination of glomerular size-selectivity in the normal rat with Ficoll. J Am Soc Nephrol. 1992 Aug;3(2):214–228. doi: 10.1681/ASN.V32214. [DOI] [PubMed] [Google Scholar]
- Robinson G. B., Walton H. A. Glomerular basement membrane as a compressible ultrafilter. Microvasc Res. 1989 Jul;38(1):36–48. doi: 10.1016/0026-2862(89)90015-0. [DOI] [PubMed] [Google Scholar]
- Takami H., Naramoto A., Shigematsu H., Ohno S. Ultrastructure of glomerular basement membrane by quick-freeze and deep-etch methods. Kidney Int. 1991 Apr;39(4):659–664. doi: 10.1038/ki.1991.79. [DOI] [PubMed] [Google Scholar]
- Walton H. A., Byrne J., Robinson G. B. Studies of the permeation properties of glomerular basement membrane: cross-linking renders glomerular basement membrane permeable to protein. Biochim Biophys Acta. 1992 Mar 20;1138(3):173–183. doi: 10.1016/0925-4439(92)90035-l. [DOI] [PubMed] [Google Scholar]


