Abstract
Addition of either bee venom or Trimeresurus flavoviridis phospholipase A2 (PLA2) to the solution bathing the front side of a voltage-clamped, planar lipid bilayer consistently produced a transitory current lasting approximately 100 s. This current is consistent with anions moving through the membrane to the rear side. The peak current is independent of holding potential. PLA2 activity on phospholipid membranes not only produced a current but also led to membrane rupture within 300 s. The current depends on Ca2+ and lipid type. Addition of PLA2 in the absence of Ca2+ or to membranes made of nonsubstrate lipids (e.g., glycerol monooleate or lysophosphatidylcholine) produced no current and did not break the bilayer. Peak current height, signal decay time, and time to membrane rupture all depended on PLA2 dose, whereas total charge produced was constant. This current does not flow through ion channels because there are no channels present and the current is not voltage dependent. The evidence is consistent with the hypothesis that the current is generated by the movement of ionized fatty acid produced by PLA2 action. These results demonstrate a simple method to measure enzyme activity in the presence of different substrates and varied environmental conditions.
Full text
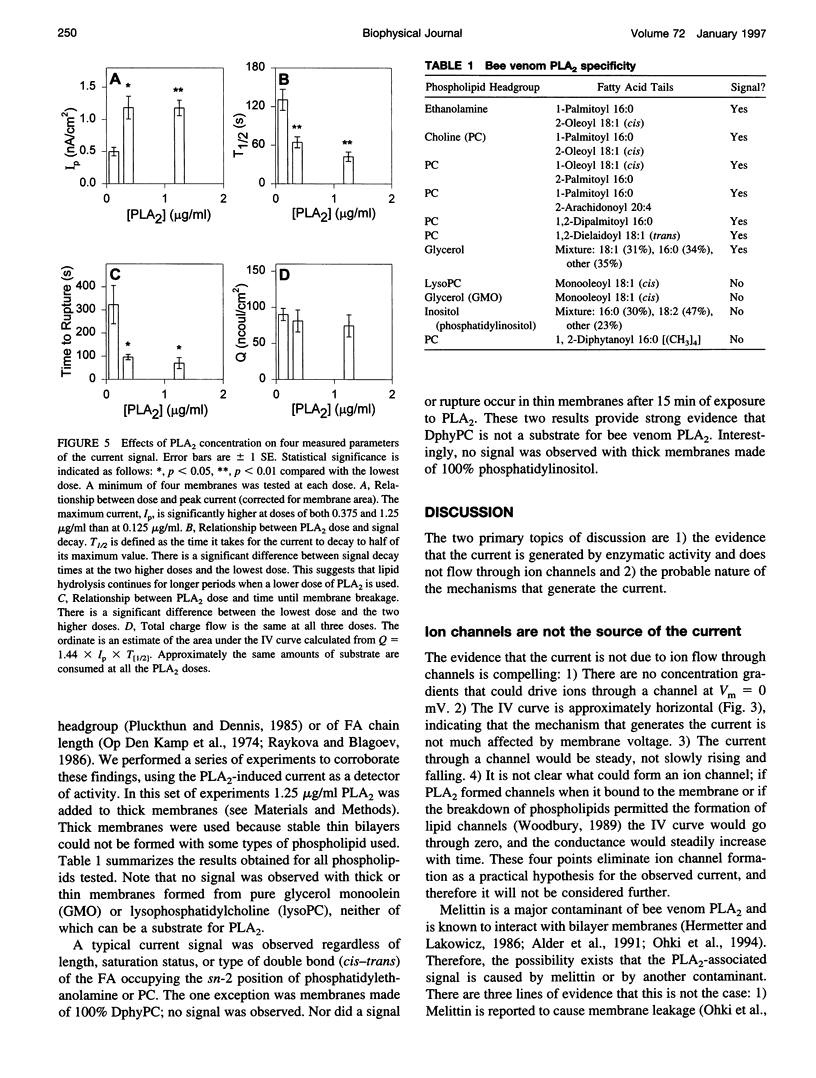
PDF

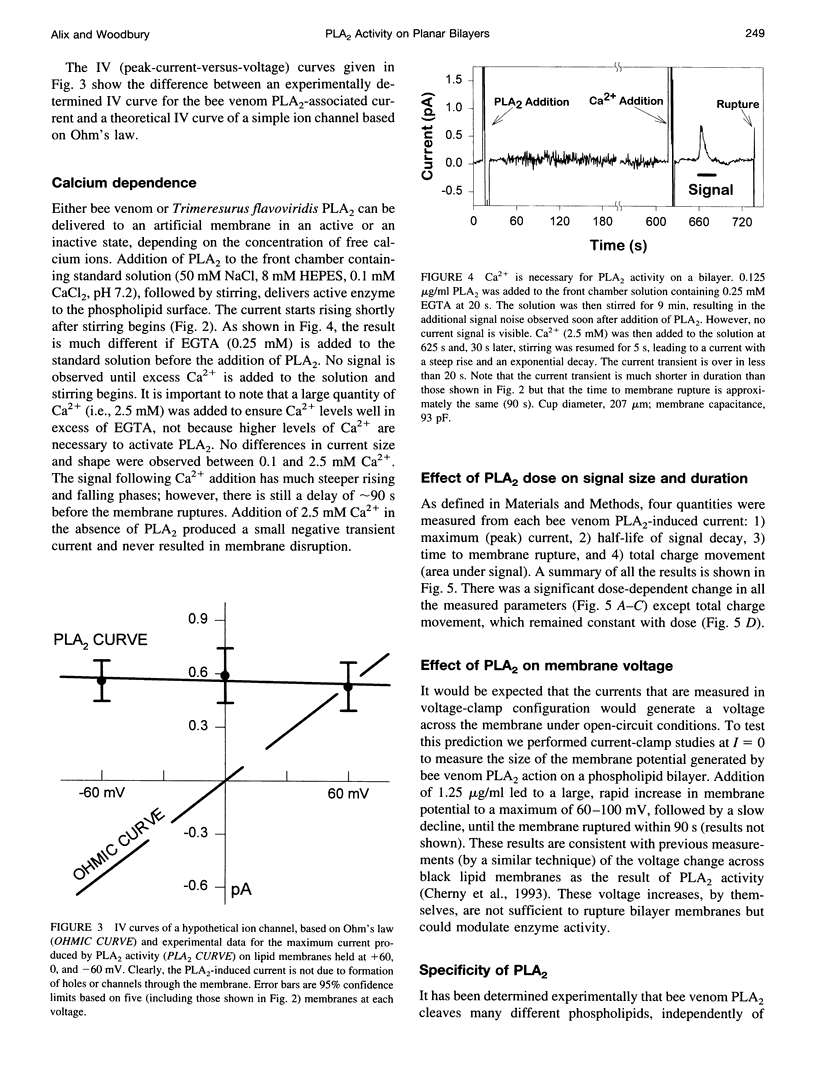


Images in this article
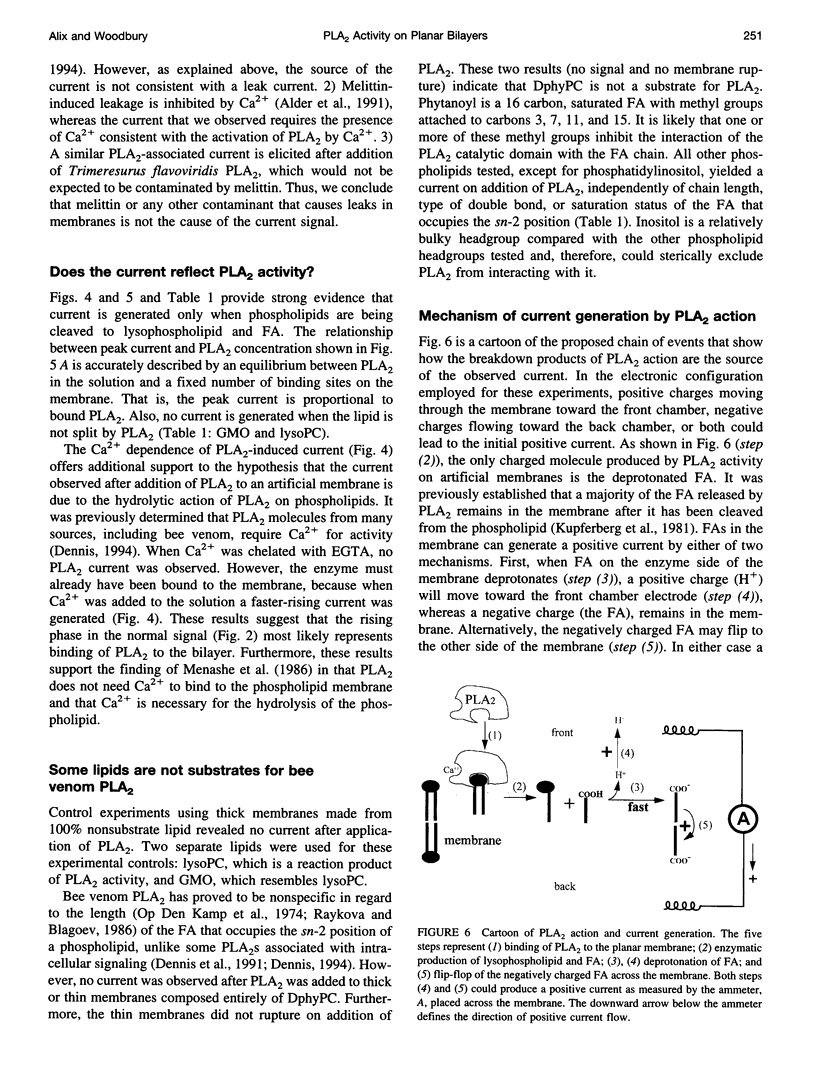
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alder G. M., Arnold W. M., Bashford C. L., Drake A. F., Pasternak C. A., Zimmermann U. Divalent cation-sensitive pores formed by natural and synthetic melittin and by Triton X-100. Biochim Biophys Acta. 1991 Jan 9;1061(1):111–120. doi: 10.1016/0005-2736(91)90275-d. [DOI] [PubMed] [Google Scholar]
- Anderson B. O., Moore E. E., Banerjee A. Phospholipase A2 regulates critical inflammatory mediators of multiple organ failure. J Surg Res. 1994 Feb;56(2):199–205. doi: 10.1006/jsre.1994.1032. [DOI] [PubMed] [Google Scholar]
- Bazan N. G., Rodriguez de Turco E. B., Allan G. Mediators of injury in neurotrauma: intracellular signal transduction and gene expression. J Neurotrauma. 1995 Oct;12(5):791–814. doi: 10.1089/neu.1995.12.791. [DOI] [PubMed] [Google Scholar]
- Cornell B. A., Separovic F. Membrane thickness and acyl chain length. Biochim Biophys Acta. 1983 Aug 24;733(1):189–193. doi: 10.1016/0005-2736(83)90106-2. [DOI] [PubMed] [Google Scholar]
- Dennis E. A., Rhee S. G., Billah M. M., Hannun Y. A. Role of phospholipase in generating lipid second messengers in signal transduction. FASEB J. 1991 Apr;5(7):2068–2077. doi: 10.1096/fasebj.5.7.1901288. [DOI] [PubMed] [Google Scholar]
- Exton J. H. Phosphoinositide phospholipases and G proteins in hormone action. Annu Rev Physiol. 1994;56:349–369. doi: 10.1146/annurev.ph.56.030194.002025. [DOI] [PubMed] [Google Scholar]
- Gips S. J., Kandzari D. E., Goldschmidt-Clermont P. J. Growth factor receptors, phospholipases, phospholipid kinases and actin reorganization. Semin Cell Biol. 1994 Jun;5(3):201–208. doi: 10.1006/scel.1994.1025. [DOI] [PubMed] [Google Scholar]
- Gutknecht J. Proton conductance caused by long-chain fatty acids in phospholipid bilayer membranes. J Membr Biol. 1988 Nov;106(1):83–93. doi: 10.1007/BF01871769. [DOI] [PubMed] [Google Scholar]
- Hermetter A., Lakowicz J. R. The aggregation state of mellitin in lipid bilayers. An energy transfer study. J Biol Chem. 1986 Jun 25;261(18):8243–8248. [PubMed] [Google Scholar]
- Huang C., Mason J. T. Geometric packing constraints in egg phosphatidylcholine vesicles. Proc Natl Acad Sci U S A. 1978 Jan;75(1):308–310. doi: 10.1073/pnas.75.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain M. K., Gelb M. H., Rogers J., Berg O. G. Kinetic basis for interfacial catalysis by phospholipase A2. Methods Enzymol. 1995;249:567–614. doi: 10.1016/0076-6879(95)49049-3. [DOI] [PubMed] [Google Scholar]
- Kelly M. L., Woodbury D. J. Ion channels from synaptic vesicle membrane fragments reconstituted into lipid bilayers. Biophys J. 1996 Jun;70(6):2593–2599. doi: 10.1016/S0006-3495(96)79830-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupferberg J. P., Yokoyama S., Kézdy F. J. The kinetics of the phospholipase A2-catalyzed hydrolysis of Egg phosphatidylcholine in unilamellar vesicles. Product inhibition and its relief by serum albumin. J Biol Chem. 1981 Jun 25;256(12):6274–6281. [PubMed] [Google Scholar]
- Lawrence A. J. Lysolecithin inhibits an action of bee venom phospholipase A2 in erythrocyte membrane. FEBS Lett. 1975 Oct 15;58(1):186–189. doi: 10.1016/0014-5793(75)80255-9. [DOI] [PubMed] [Google Scholar]
- Menashe M., Romero G., Biltonen R. L., Lichtenberg D. Hydrolysis of dipalmitoylphosphatidylcholine small unilamellar vesicles by porcine pancreatic phospholipase A2. J Biol Chem. 1986 Apr 25;261(12):5328–5333. [PubMed] [Google Scholar]
- Miyazaki J., Hideg K., Marsh D. Interfacial ionization and partitioning of membrane-bound local anesthetics. Biochim Biophys Acta. 1992 Jan 10;1103(1):62–68. doi: 10.1016/0005-2736(92)90057-s. [DOI] [PubMed] [Google Scholar]
- Navab M., Fogelman A. M., Berliner J. A., Territo M. C., Demer L. L., Frank J. S., Watson A. D., Edwards P. A., Lusis A. J. Pathogenesis of atherosclerosis. Am J Cardiol. 1995 Sep 28;76(9):18C–23C. doi: 10.1016/s0002-9149(99)80466-4. [DOI] [PubMed] [Google Scholar]
- O'Regan M. H., Alix S., Woodbury D. J. Phospholipase A2-evoked destabilization of planar lipid membranes. Neurosci Lett. 1996 Jan 5;202(3):201–203. doi: 10.1016/0304-3940(95)12238-9. [DOI] [PubMed] [Google Scholar]
- O'Regan M. H., Smith-Barbour M., Perkins L. M., Phillis J. W. A possible role for phospholipases in the release of neurotransmitter amino acids from ischemic rat cerebral cortex. Neurosci Lett. 1995 Feb 13;185(3):191–194. doi: 10.1016/0304-3940(95)11259-y. [DOI] [PubMed] [Google Scholar]
- Ohki S., Marcus E., Sukumaran D. K., Arnold K. Interaction of melittin with lipid membranes. Biochim Biophys Acta. 1994 Sep 14;1194(2):223–232. doi: 10.1016/0005-2736(94)90303-4. [DOI] [PubMed] [Google Scholar]
- Op den Kamp J. A., de Gier J., van Deenen L. L. Hydrolysis of phosphatidylcholine liposomes by pancreatic phospholipase A2 at the transition temperature. Biochim Biophys Acta. 1974 Apr 29;345(2):253–256. doi: 10.1016/0005-2736(74)90263-6. [DOI] [PubMed] [Google Scholar]
- Plückthun A., Dennis E. A. Activation, aggregation, and product inhibition of cobra venom phospholipase A2 and comparison with other phospholipases. J Biol Chem. 1985 Sep 15;260(20):11099–11106. [PubMed] [Google Scholar]
- Raykova D., Blagoev B. Hydrolysis of short-chain phosphatidylcholines by bee venom phospholipase A2. Toxicon. 1986;24(8):791–797. doi: 10.1016/0041-0101(86)90104-2. [DOI] [PubMed] [Google Scholar]
- Sheffield M. J., Baker B. L., Li D., Owen N. L., Baker M. L., Bell J. D. Enhancement of Agkistrodon piscivorus piscivorus venom phospholipase A2 activity toward phosphatidylcholine vesicles by lysolecithin and palmitic acid: studies with fluorescent probes of membrane structure. Biochemistry. 1995 Jun 20;34(24):7796–7806. doi: 10.1021/bi00024a003. [DOI] [PubMed] [Google Scholar]
- Verheij H. M., Slotboom A. J., de Haas G. H. Structure and function of phospholipase A2. Rev Physiol Biochem Pharmacol. 1981;91:91–203. doi: 10.1007/3-540-10961-7_3. [DOI] [PubMed] [Google Scholar]
- Woodbury D. J. Pure lipid vesicles can induce channel-like conductances in planar bilayers. J Membr Biol. 1989 Jul;109(2):145–150. doi: 10.1007/BF01870853. [DOI] [PubMed] [Google Scholar]