Abstract
Semiautomatic single-axis tilt electron tomography has been used to visualize the three-dimensional organization of actin filaments in "phantom cells," i.e. lipid vesicles. The instrumentation consisted of a 120-kV electron microscope equipped with a postcolumn energy filter, which was used in the zero-loss imaging mode. Apart from changing the tilt angle, all steps required for automated tomography, such as recentering the image area, refocusing, and centering the energy-selecting slit, were performed by external computer control. This setup permitted imaging of ice-embedded samples up to a thickness of 800 nm with improved image contrast compared with that produced by tomography with a conventional electron microscope. In spite of the missing-wedge effect that is especially obvious in the study of membrane-filament interaction, single-axis tilt tomography was found to be an appropriate (in fact the only available) method for this kind of investigation. In contrast to random actin networks found in actin gels, actin filaments in and on vesicles with a bending radius of less than approximately 2 microns tend to be arranged in single layers of parallel filaments and often induce an elongated shape of the vesicles. Actin filaments located on the outside usually associate with the vesicle membrane.
Full text
PDF

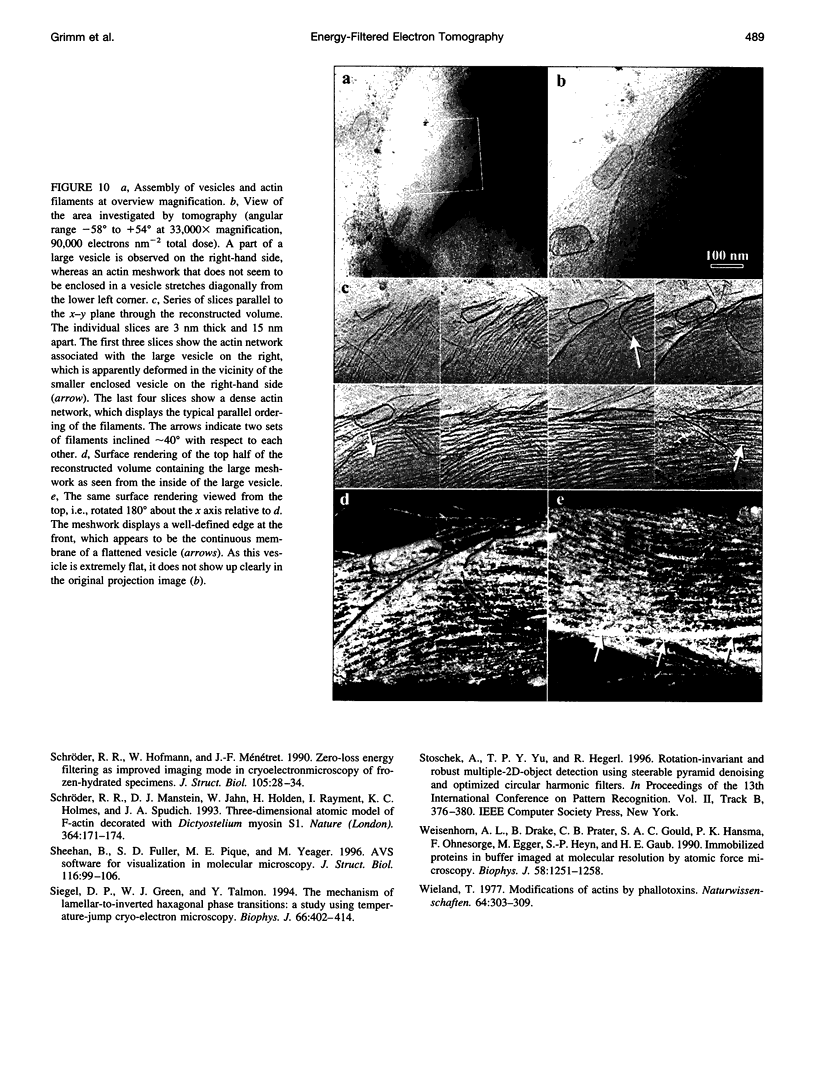
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Conway J. F., Trus B. L., Booy F. P., Newcomb W. W., Brown J. C., Steven A. C. The effects of radiation damage on the structure of frozen hydrated HSV-1 capsids. J Struct Biol. 1993 Nov-Dec;111(3):222–233. doi: 10.1006/jsbi.1993.1052. [DOI] [PubMed] [Google Scholar]
- Cortese J. D., Schwab B., 3rd, Frieden C., Elson E. L. Actin polymerization induces a shape change in actin-containing vesicles. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5773–5777. doi: 10.1073/pnas.86.15.5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierksen K., Typke D., Hegerl R., Walz J., Sackmann E., Baumeister W. Three-dimensional structure of lipid vesicles embedded in vitreous ice and investigated by automated electron tomography. Biophys J. 1995 Apr;68(4):1416–1422. doi: 10.1016/S0006-3495(95)80314-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank J., Zhu J., Penczek P., Li Y., Srivastava S., Verschoor A., Radermacher M., Grassucci R., Lata R. K., Agrawal R. K. A model of protein synthesis based on cryo-electron microscopy of the E. coli ribosome. Nature. 1995 Aug 3;376(6539):441–444. doi: 10.1038/376441a0. [DOI] [PubMed] [Google Scholar]
- Grimm R., Typke D., Bärmann M., Baumeister W. Determination of the inelastic mean free path in ice by examination of tilted vesicles and automated most probable loss imaging. Ultramicroscopy. 1996 Jul;63(3-4):169–179. doi: 10.1016/0304-3991(96)00035-6. [DOI] [PubMed] [Google Scholar]
- Hegerl R. The EM Program Package: A Platform for Image Processing in Biological Electron Microscopy. J Struct Biol. 1996 Oct;116(1):30–34. doi: 10.1006/jsbi.1996.0006. [DOI] [PubMed] [Google Scholar]
- Krivanek O. L., Friedman S. L., Gubbens A. J., Kraus B. An imaging filter for biological applications. Ultramicroscopy. 1995 Jul;59(1-4):267–282. doi: 10.1016/0304-3991(95)00034-x. [DOI] [PubMed] [Google Scholar]
- Laliberte A., Gicquaud C. Polymerization of actin by positively charged liposomes. J Cell Biol. 1988 Apr;106(4):1221–1227. doi: 10.1083/jcb.106.4.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmore J. P., Smith M. F. Quantitative energy-filtered electron microscopy of biological molecules in ice. Ultramicroscopy. 1992 Oct;46(1-4):349–373. doi: 10.1016/0304-3991(92)90024-e. [DOI] [PubMed] [Google Scholar]
- Malis T., Cheng S. C., Egerton R. F. EELS log-ratio technique for specimen-thickness measurement in the TEM. J Electron Microsc Tech. 1988 Feb;8(2):193–200. doi: 10.1002/jemt.1060080206. [DOI] [PubMed] [Google Scholar]
- O'Toole E., Wray G., Kremer J., McIntosh J. R. High voltage cryomicroscopy of human blood platelets. J Struct Biol. 1993 Jan-Feb;110(1):55–66. doi: 10.1006/jsbi.1993.1004. [DOI] [PubMed] [Google Scholar]
- Schröder R. R., Manstein D. J., Jahn W., Holden H., Rayment I., Holmes K. C., Spudich J. A. Three-dimensional atomic model of F-actin decorated with Dictyostelium myosin S1. Nature. 1993 Jul 8;364(6433):171–174. doi: 10.1038/364171a0. [DOI] [PubMed] [Google Scholar]
- Sheehan B., Fuller S. D., Pique M. E., Yeager M. AVS software for visualization in molecular microscopy. J Struct Biol. 1996 Jan-Feb;116(1):99–106. doi: 10.1006/jsbi.1996.0017. [DOI] [PubMed] [Google Scholar]
- Siegel D. P., Green W. J., Talmon Y. The mechanism of lamellar-to-inverted hexagonal phase transitions: a study using temperature-jump cryo-electron microscopy. Biophys J. 1994 Feb;66(2 Pt 1):402–414. doi: 10.1016/s0006-3495(94)80790-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisenhorn A. L., Drake B., Prater C. B., Gould S. A., Hansma P. K., Ohnesorge F., Egger M., Heyn S. P., Gaub H. E. Immobilized proteins in buffer imaged at molecular resolution by atomic force microscopy. Biophys J. 1990 Nov;58(5):1251–1258. doi: 10.1016/S0006-3495(90)82465-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland T. Modification of actins by phallotoxins. Naturwissenschaften. 1977 Jun;64(6):303–309. doi: 10.1007/BF00446784. [DOI] [PubMed] [Google Scholar]