Abstract
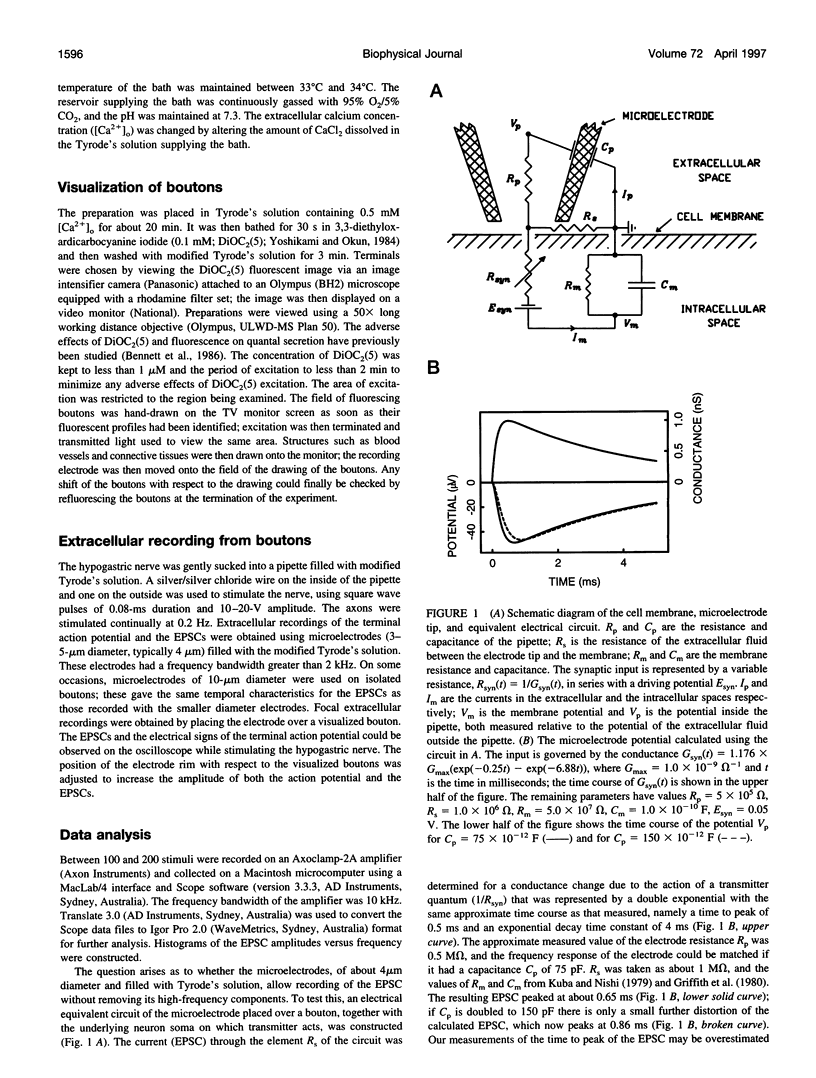
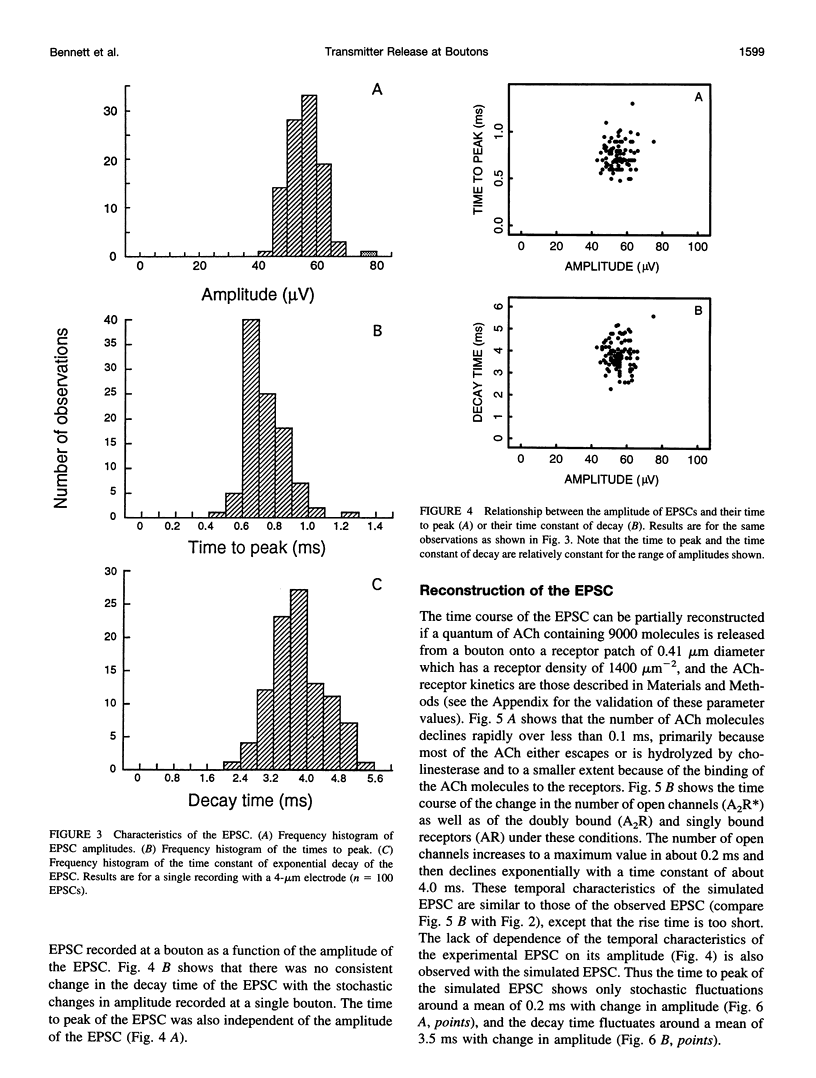
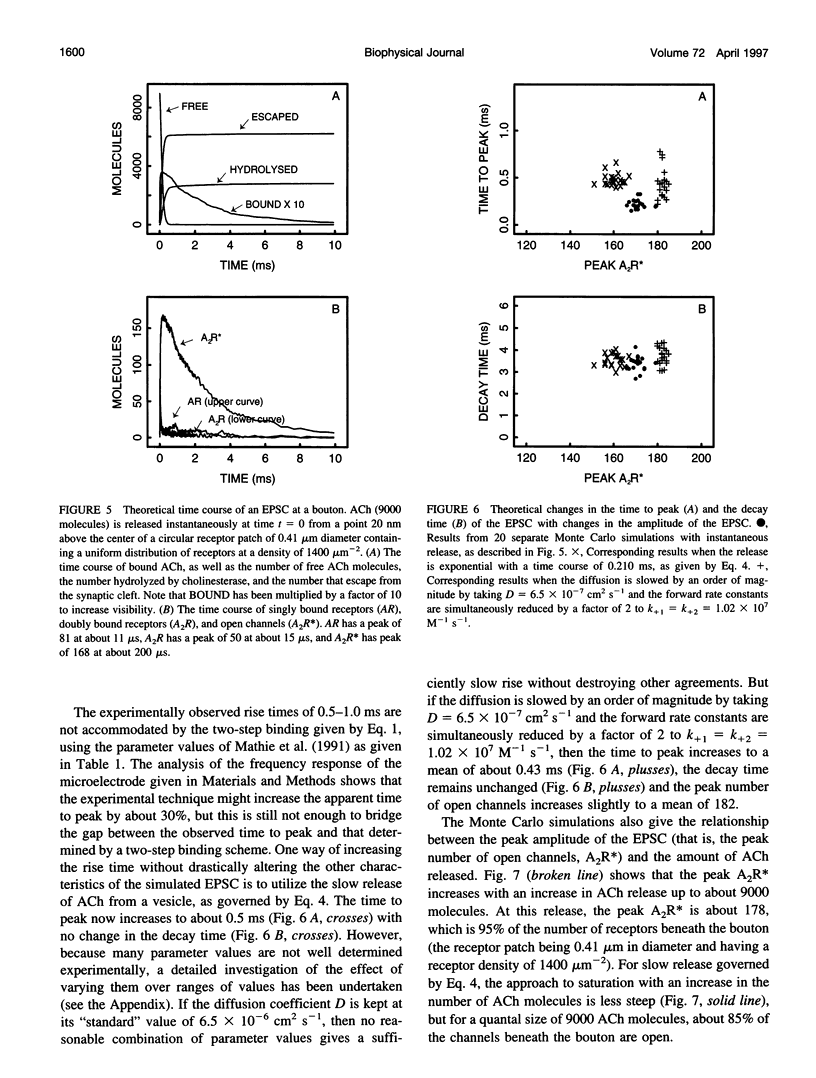
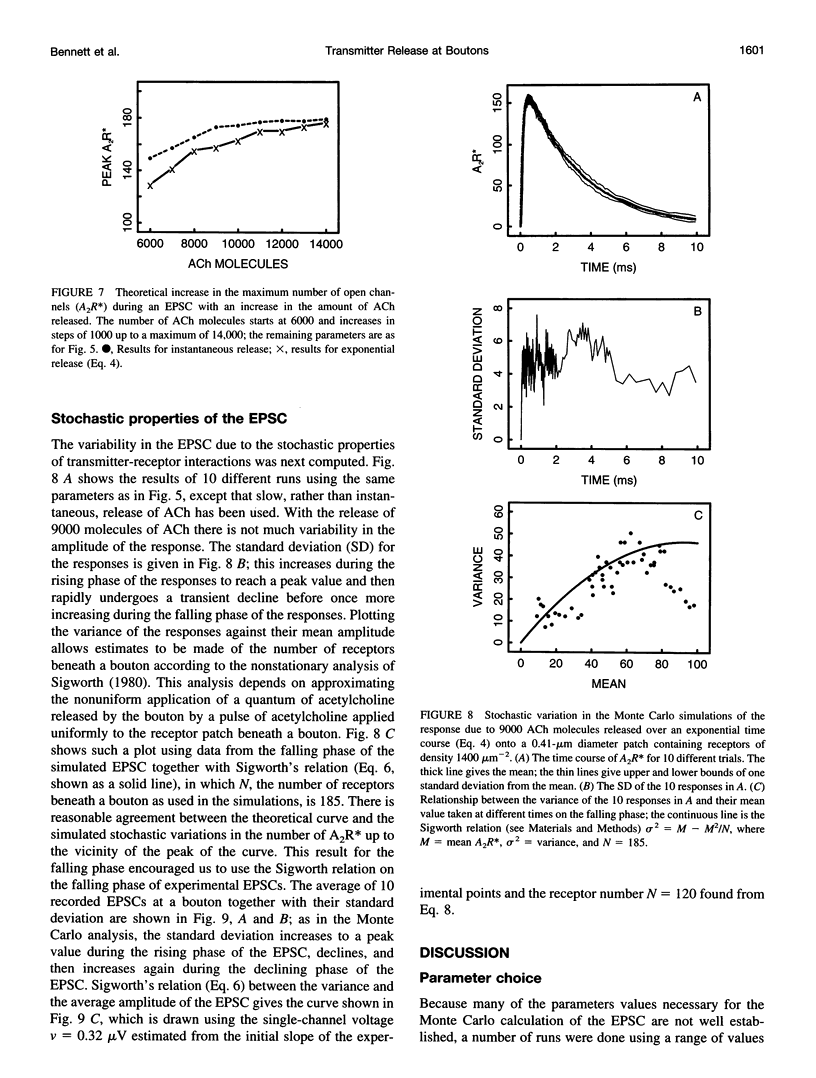
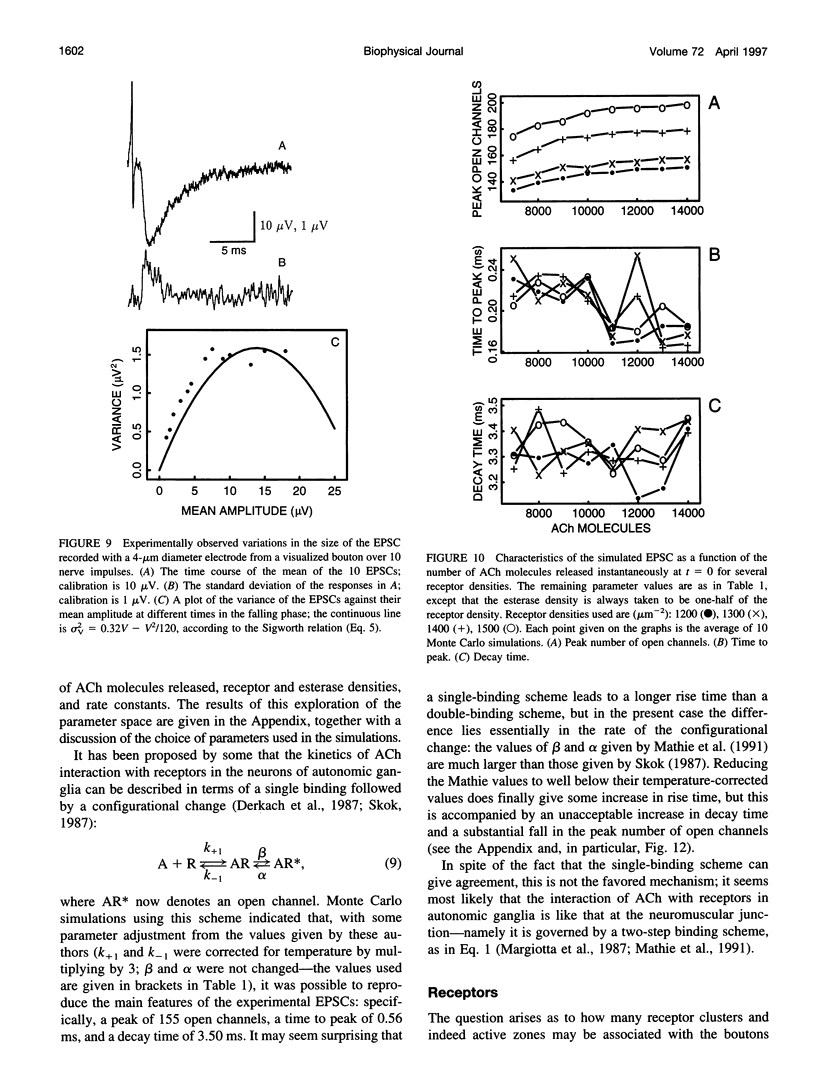
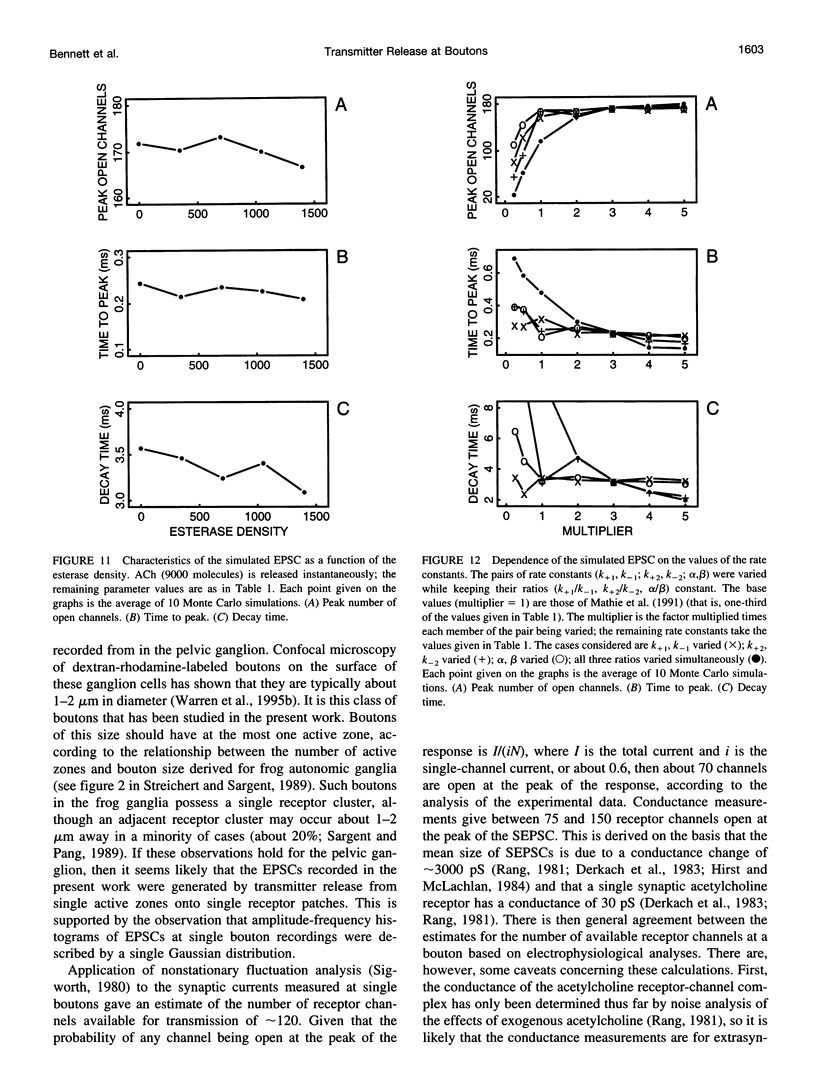
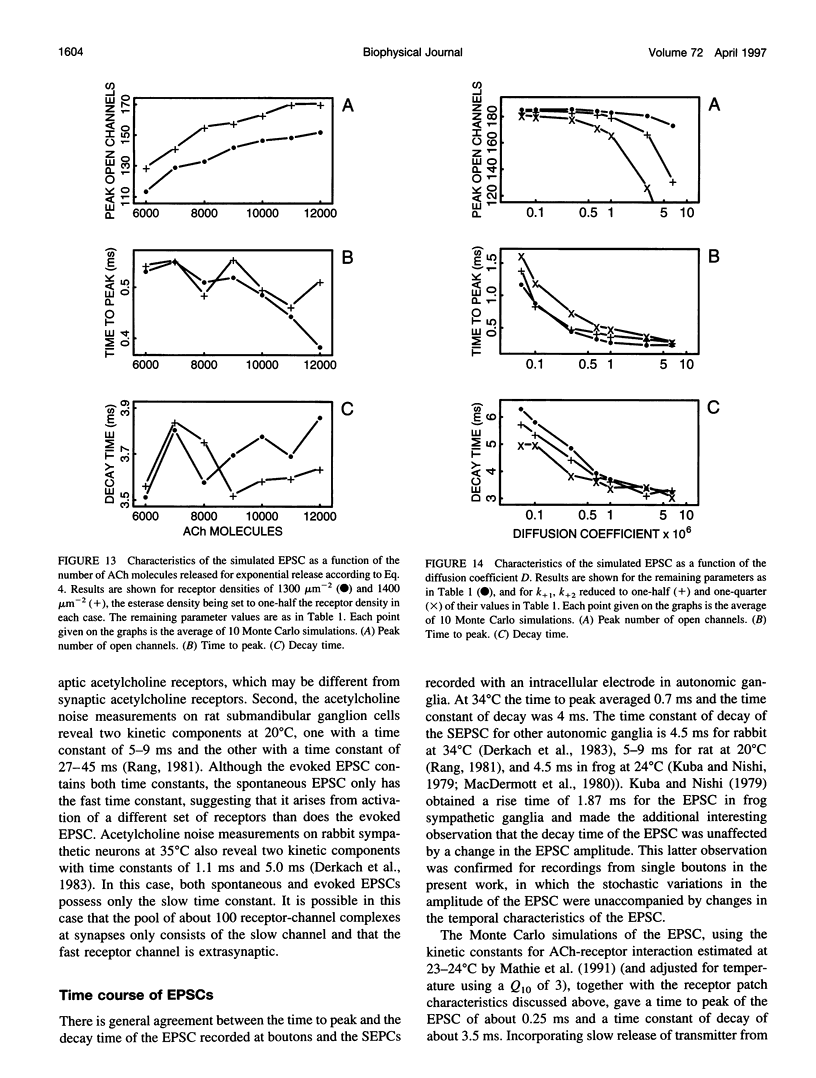
Excitatory postsynaptic currents (EPSCs) were recorded with loose patch electrodes placed over visualized boutons on the surface of rat pelvic ganglion cells. At 34 degrees C the time to peak of the EPSC was about 0.7 ms, and a single exponential described the declining phase with a time constant of about 4.0 ms; these times were not correlated with changes in the amplitude of the EPSC. The amplitude-frequency histogram of the EPSC at individual boutons was well described by a single Gaussian-distribution that possessed a variance similar to that of the electrical noise. Nonstationary fluctuation analysis of the EPSCs at a bouton indicated that about 120 ACh receptor channels were available beneath boutons for interaction with a quantum of ACh. The characteristics of these EPSCs were compared with the results of Monte Carlo simulations of the quantal release of 9000 acetylcholine (ACh) molecules onto receptor patches of density 1400 microns-2 and 0.41 micron diameter, using a kinetic scheme of interaction between ACh and the receptors similar to that observed at the neuromuscular junction. The simulated EPSC generated in this way had temporal characteristics similar to those of the experimental EPSC when either the diffusion of the ACh is slowed or allowance is made for a finite period of transmitter release from the bouton. The amplitude of the simulated EPSC then exhibited stochastic fluctuations similar to those of the experimental EPSC.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anglister L., Stiles J. R., Salpeter M. M. Acetylcholinesterase density and turnover number at frog neuromuscular junctions, with modeling of their role in synaptic function. Neuron. 1994 Apr;12(4):783–794. doi: 10.1016/0896-6273(94)90331-x. [DOI] [PubMed] [Google Scholar]
- Bartol T. M., Jr, Land B. R., Salpeter E. E., Salpeter M. M. Monte Carlo simulation of miniature endplate current generation in the vertebrate neuromuscular junction. Biophys J. 1991 Jun;59(6):1290–1307. doi: 10.1016/S0006-3495(91)82344-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Farnell L., Gibson W. G., Karunanithi S. Quantal transmission at purinergic junctions: stochastic interaction between ATP and its receptors. Biophys J. 1995 Mar;68(3):925–935. doi: 10.1016/S0006-3495(95)80268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Gibson W. G., Robinson J. Probabilistic secretion of quanta: spontaneous release at active zones of varicosities, boutons, and endplates. Biophys J. 1995 Jul;69(1):42–56. doi: 10.1016/S0006-3495(95)79873-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Jones P., Lavidis N. A. The probability of quantal secretion along visualized terminal branches at amphibian (Bufo marinus) neuromuscular synapses. J Physiol. 1986 Oct;379:257–274. doi: 10.1113/jphysiol.1986.sp016252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R. The origin of Gaussian distributions of synaptic potentials. Prog Neurobiol. 1995 Jul;46(4):331–350. doi: 10.1016/0301-0082(94)00061-l. [DOI] [PubMed] [Google Scholar]
- Betz W. J., Mao F., Bewick G. S. Activity-dependent fluorescent staining and destaining of living vertebrate motor nerve terminals. J Neurosci. 1992 Feb;12(2):363–375. doi: 10.1523/JNEUROSCI.12-02-00363.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein J. C. Spontaneous multiquantal release at synapses in guinea-pig hypogastric ganglia: evidence that release can occur in bursts. J Physiol. 1978 Sep;282:375–398. doi: 10.1113/jphysiol.1978.sp012470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch C., Sakmann B. Synaptic transmission in hippocampal neurons: numerical reconstruction of quantal IPSCs. Cold Spring Harb Symp Quant Biol. 1990;55:69–80. doi: 10.1101/sqb.1990.055.01.009. [DOI] [PubMed] [Google Scholar]
- Derkach V. A., North R. A., Selyanko A. A., Skok V. I. Single channels activated by acetylcholine in rat superior cervical ganglion. J Physiol. 1987 Jul;388:141–151. doi: 10.1113/jphysiol.1987.sp016606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkach V. A., Selyanko A. A., Skok V. I. Acetylcholine-induced current fluctuations and fast excitatory post-synaptic currents in rabbit sympathetic neurones. J Physiol. 1983 Mar;336:511–526. doi: 10.1113/jphysiol.1983.sp014595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryer S. E., Chiappinelli V. A. Analysis of quantal content and quantal conductance in two populations of neurons in the avian ciliary ganglion. Neuroscience. 1987 Mar;20(3):905–910. doi: 10.1016/0306-4522(87)90251-x. [DOI] [PubMed] [Google Scholar]
- Edwards F. Neurobiology. LTP is a long term problem. Nature. 1991 Mar 28;350(6316):271–272. doi: 10.1038/350271a0. [DOI] [PubMed] [Google Scholar]
- Griffith W. H., 3rd, Gallagher J. P., Shinnick-Gallagher P. An intracellular investigation of cat vesical pelvic ganglia. J Neurophysiol. 1980 Feb;43(2):343–354. doi: 10.1152/jn.1980.43.2.343. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., McLachlan E. M. Post-natal development of ganglia in the lower lumbar sympathetic chain of the rat. J Physiol. 1984 Apr;349:119–134. doi: 10.1113/jphysiol.1984.sp015147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanin R., Parnas H., Segel L. Diffusion cannot govern the discharge of neurotransmitter in fast synapses. Biophys J. 1994 Sep;67(3):966–972. doi: 10.1016/S0006-3495(94)80562-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K., Nishi S. Characteristics of fast excitatory postsynaptic current in bullfrog sympathetic ganglion cells. Effects of membrane potential, temperature and Ca ions. Pflugers Arch. 1979 Jan 31;378(3):205–212. doi: 10.1007/BF00592737. [DOI] [PubMed] [Google Scholar]
- Lavidis N. A., Bennett M. R. Probabilistic secretion of quanta from visualized sympathetic nerve varicosities in mouse vas deferens. J Physiol. 1992 Aug;454:9–26. doi: 10.1113/jphysiol.1992.sp019252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loring R. H., Sah D. W., Landis S. C., Zigmond R. E. The ultrastructural distribution of putative nicotinic receptors on cultured neurons from the rat superior cervical ganglion. Neuroscience. 1988 Mar;24(3):1071–1080. doi: 10.1016/0306-4522(88)90088-7. [DOI] [PubMed] [Google Scholar]
- Loring R. H., Zigmond R. E. Ultrastructural distribution of 125I-toxin F binding sites on chick ciliary neurons: synaptic localization of a toxin that blocks ganglionic nicotinic receptors. J Neurosci. 1987 Jul;7(7):2153–2162. doi: 10.1523/JNEUROSCI.07-07-02153.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A. R., PILAR G. QUANTAL COMPONENTS OF THE SYNAPTIC POTENTIAL IN THE CILIARY GANGLION OF THE CHICK. J Physiol. 1964 Dec;175:1–16. doi: 10.1113/jphysiol.1964.sp007499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDermott A. B., Connor E. A., Dionne V. E., Parsons R. L. Voltage clamp study of fast excitatory synaptic currents in bullfrog sympathetic ganglion cells. J Gen Physiol. 1980 Jan;75(1):39–60. doi: 10.1085/jgp.75.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margiotta J. F., Berg D. K., Dionne V. E. The properties and regulation of functional acetylcholine receptors on chick ciliary ganglion neurons. J Neurosci. 1987 Nov;7(11):3612–3622. doi: 10.1523/JNEUROSCI.07-11-03612.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathie A., Cull-Candy S. G., Colquhoun D. Conductance and kinetic properties of single nicotinic acetylcholine receptor channels in rat sympathetic neurones. J Physiol. 1991 Aug;439:717–750. doi: 10.1113/jphysiol.1991.sp018690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews-Bellinger J., Salpeter M. M. Distribution of acetylcholine receptors at frog neuromuscular junctions with a discussion of some physiological implications. J Physiol. 1978 Jun;279:197–213. doi: 10.1113/jphysiol.1978.sp012340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Molenaar P. C., Polak R. L., Tas J. W., van der Laaken T. Neural and non-neural acetylcholine in the rat diaphragm. Proc R Soc Lond B Biol Sci. 1982 Jan 22;214(1195):153–168. doi: 10.1098/rspb.1982.0002. [DOI] [PubMed] [Google Scholar]
- Rang H. P. The characteristics of synaptic currents and responses to acetylcholine of rat submandibular ganglion cells. J Physiol. 1981 Feb;311:23–55. doi: 10.1113/jphysiol.1981.sp013571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent P. B., Pang D. Z. Acetylcholine receptor-like molecules are found in both synaptic and extrasynaptic clusters on the surface of neurons in the frog cardiac ganglion. J Neurosci. 1989 Mar;9(3):1062–1072. doi: 10.1523/JNEUROSCI.09-03-01062.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent P. B., Pang D. Z. Denervation alters the size, number, and distribution of clusters of acetylcholine receptor-like molecules on frog cardiac ganglion neurons. Neuron. 1988 Nov;1(9):877–886. doi: 10.1016/0896-6273(88)90135-3. [DOI] [PubMed] [Google Scholar]
- Sargent P. B. The diversity of neuronal nicotinic acetylcholine receptors. Annu Rev Neurosci. 1993;16:403–443. doi: 10.1146/annurev.ne.16.030193.002155. [DOI] [PubMed] [Google Scholar]
- Sigworth F. J. The variance of sodium current fluctuations at the node of Ranvier. J Physiol. 1980 Oct;307:97–129. doi: 10.1113/jphysiol.1980.sp013426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skok V. I. Nicotinic acetylcholine receptors in the neurones of autonomic ganglia. J Auton Nerv Syst. 1987 Dec;21(2-3):91–99. doi: 10.1016/0165-1838(87)90012-9. [DOI] [PubMed] [Google Scholar]
- Stiles J. R., Van Helden D., Bartol T. M., Jr, Salpeter E. E., Salpeter M. M. Miniature endplate current rise times less than 100 microseconds from improved dual recordings can be modeled with passive acetylcholine diffusion from a synaptic vesicle. Proc Natl Acad Sci U S A. 1996 Jun 11;93(12):5747–5752. doi: 10.1073/pnas.93.12.5747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streichert L. C., Sargent P. B. Bouton ultrastructure and synaptic growth in a frog autonomic ganglion. J Comp Neurol. 1989 Mar 1;281(1):159–168. doi: 10.1002/cne.902810113. [DOI] [PubMed] [Google Scholar]
- Tabatai M., Booth A. M., de Groat W. C. Morphological and electrophysiological properties of pelvic ganglion cells in the rat. Brain Res. 1986 Sep 10;382(1):61–70. doi: 10.1016/0006-8993(86)90111-3. [DOI] [PubMed] [Google Scholar]
- Van der Kloot W. The rise times of miniature endplate currents suggest that acetylcholine may be released over a period of time. Biophys J. 1995 Jul;69(1):148–154. doi: 10.1016/S0006-3495(95)79884-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren D., Lavidis N. A., Bennett M. R. Quantal secretion from visualized boutons on rat pelvic ganglion neurones. J Auton Nerv Syst. 1996 Jan 5;56(3):175–183. doi: 10.1016/0165-1838(95)00087-9. [DOI] [PubMed] [Google Scholar]
- Warren D., Lavidis N. A., Bennett M. R. Quantal secretion recorded from visualized boutons. Neurosci Lett. 1995 Jun 16;192(3):205–208. doi: 10.1016/0304-3940(95)11646-e. [DOI] [PubMed] [Google Scholar]
- Whittaker V. P. Cholinergic synaptic vesicles are metabolically and biophysically heterogeneous even in resting terminals. Brain Res. 1990 Mar 12;511(1):113–121. doi: 10.1016/0006-8993(90)90230-9. [DOI] [PubMed] [Google Scholar]
- Yokota R., Burnstock G. Synaptic organisation of the pelvic ganglion in the guinea-pig. Cell Tissue Res. 1983;232(2):379–397. doi: 10.1007/BF00213794. [DOI] [PubMed] [Google Scholar]
- Yoshikami D., Okun L. M. Staining of living presynaptic nerve terminals with selective fluorescent dyes. Nature. 1984 Jul 5;310(5972):53–56. doi: 10.1038/310053a0. [DOI] [PubMed] [Google Scholar]


