Abstract
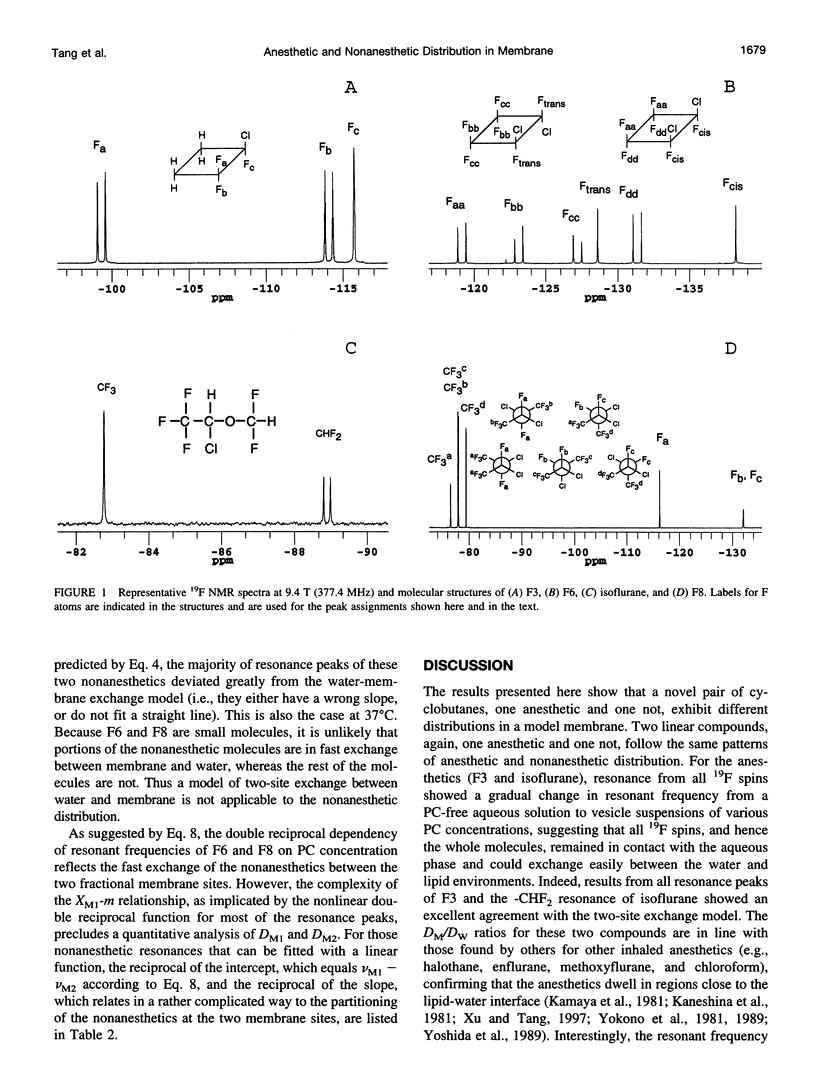
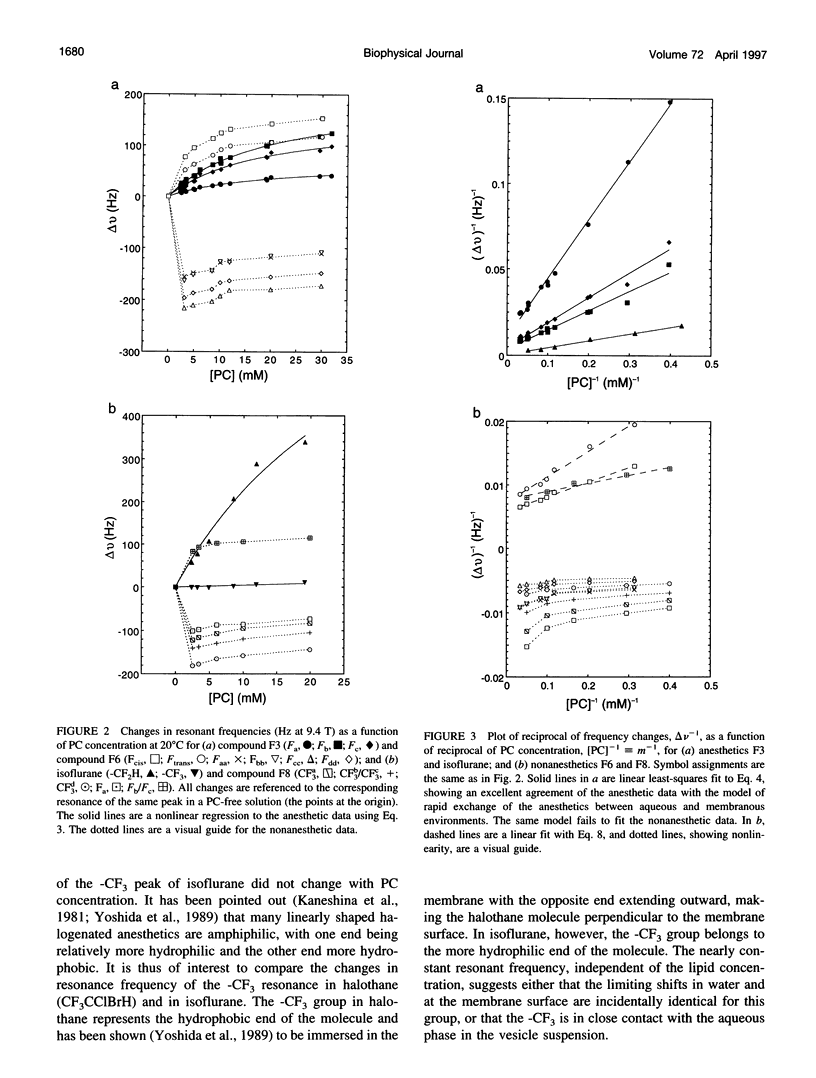
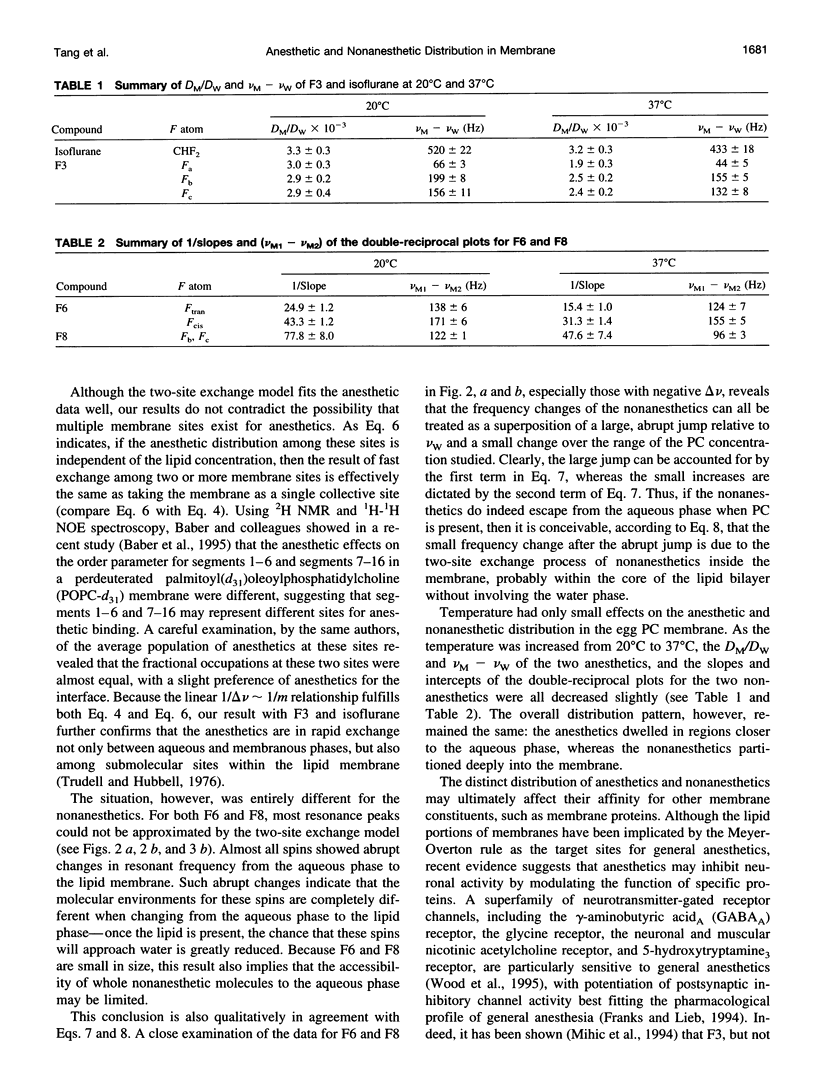
Despite their structural resemblance, a pair of cyclic halogenated compounds, 1-chloro-1,2,2-trifluorocyclobutane (F3) and 1,2-dichlorohexafluorocyclobutane (F6), exhibit completely different anesthetic properties. Whereas the former is a potent general anesthetic, the latter produces no anesthesia. Two linear compounds, isoflurane and 2,3-dichlorooctofluorobutane (F8), although not a structural pair, also show the same anesthetic discrepancy. Using 19F nuclear magnetic spectroscopy, we investigated the time-averaged submolecular distribution of these compounds in a vesicle suspension of phosphatidylcholine lipids. A two-site exchange model was used to interpret the observed changes in resonance frequencies as a function of the solubilization of these compounds in membrane and in water. At clinically relevant concentrations, the anesthetics F3 and isoflurane distributed preferentially to regions of the membrane that permit easy contact with water. The frequency changes of these two anesthetics can be well characterized by the two-site exchange model. In contrast, the nonanesthetics F6 and F8 solubilized deeply into the lipid core, and their frequency change significantly deviated from the prediction of the model. It is concluded that although anesthetics and nonanesthetics may show similar hydrophobicity in bulk solvents such as olive oil, their distributions in various regions in biomembranes, and hence their effective concentrations at different submolecular sites, may differ significantly.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alifimoff J. K., Firestone L. L., Miller K. W. Anaesthetic potencies of primary alkanols: implications for the molecular dimensions of the anaesthetic site. Br J Pharmacol. 1989 Jan;96(1):9–16. doi: 10.1111/j.1476-5381.1989.tb11777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baber J., Ellena J. F., Cafiso D. S. Distribution of general anesthetics in phospholipid bilayers determined using 2H NMR and 1H-1H NOE spectroscopy. Biochemistry. 1995 May 16;34(19):6533–6539. doi: 10.1021/bi00019a035. [DOI] [PubMed] [Google Scholar]
- Dubois B. W., Evers A. S. 19F-NMR spin-spin relaxation (T2) method for characterizing volatile anesthetic binding to proteins. Analysis of isoflurane binding to serum albumin. Biochemistry. 1992 Aug 11;31(31):7069–7076. doi: 10.1021/bi00146a007. [DOI] [PubMed] [Google Scholar]
- Eger E. I., 2nd, Liu J., Koblin D. D., Laster M. J., Taheri S., Halsey M. J., Ionescu P., Chortkoff B. S., Hudlicky T. Molecular properties of the "ideal" inhaled anesthetic: studies of fluorinated methanes, ethanes, propanes, and butanes. Anesth Analg. 1994 Aug;79(2):245–251. doi: 10.1213/00000539-199408000-00007. [DOI] [PubMed] [Google Scholar]
- Fraenkel Y., Navon G., Aronheim A., Gershoni J. M. Direct measurement of agonist binding to genetically engineered peptides of the acetylcholine receptor by selective T1 NMR relaxation. Biochemistry. 1990 Mar 13;29(10):2617–2622. doi: 10.1021/bi00462a027. [DOI] [PubMed] [Google Scholar]
- Franks N. P., Lieb W. R. Molecular and cellular mechanisms of general anaesthesia. Nature. 1994 Feb 17;367(6464):607–614. doi: 10.1038/367607a0. [DOI] [PubMed] [Google Scholar]
- Kamaya H., Kaneshina S., Ueda I. Partition equilibrium of inhalation anesthetics and alcohols between water and membranes of phospholipids with varying acyl chain-lengths. Biochim Biophys Acta. 1981 Aug 6;646(1):135–142. doi: 10.1016/0005-2736(81)90280-7. [DOI] [PubMed] [Google Scholar]
- Kaneshina S., Lin H. C., Ueda I. Unisotropic solubilization of an inhalation anesthetic, methoxyflurane, into the interfacial region of cationic surfactant micelles. Biochim Biophys Acta. 1981 Oct 2;647(2):223–226. doi: 10.1016/0005-2736(81)90249-2. [DOI] [PubMed] [Google Scholar]
- Koblin D. D., Chortkoff B. S., Laster M. J., Eger E. I., 2nd, Halsey M. J., Ionescu P. Polyhalogenated and perfluorinated compounds that disobey the Meyer-Overton hypothesis. Anesth Analg. 1994 Dec;79(6):1043–1048. doi: 10.1213/00000539-199412000-00004. [DOI] [PubMed] [Google Scholar]
- Liu J., Laster M. J., Koblin D. D., Eger E. I., 2nd, Halsey M. J., Taheri S., Chortkoff B. A cutoff in potency exists in the perfluoroalkanes. Anesth Analg. 1994 Aug;79(2):238–244. doi: 10.1213/00000539-199408000-00006. [DOI] [PubMed] [Google Scholar]
- Liu J., Laster M. J., Taheri S., Eger E. I., 2nd, Chortkoff B., Halsey M. J. Effect of n-alkane kinetics in rats on potency estimations and the Meyer-Overton hypothesis. Anesth Analg. 1994 Dec;79(6):1049–1055. doi: 10.1213/00000539-199412000-00005. [DOI] [PubMed] [Google Scholar]
- Mihic S. J., McQuilkin S. J., Eger E. I., 2nd, Ionescu P., Harris R. A. Potentiation of gamma-aminobutyric acid type A receptor-mediated chloride currents by novel halogenated compounds correlates with their abilities to induce general anesthesia. Mol Pharmacol. 1994 Nov;46(5):851–857. [PubMed] [Google Scholar]
- Mills P., Sessler D. I., Moseley M., Chew W., Pereira B., James T. L., Litt L. An in vivo 19F nuclear magnetic resonance study of isoflurane elimination from the rabbit brain. Anesthesiology. 1987 Aug;67(2):169–173. doi: 10.1097/00000542-198708000-00003. [DOI] [PubMed] [Google Scholar]
- Raines D. E., Korten S. E., Hill A. G., Miller K. W. Anesthetic cutoff in cycloalkanemethanols. A test of current theories. Anesthesiology. 1993 May;78(5):918–927. doi: 10.1097/00000542-199305000-00017. [DOI] [PubMed] [Google Scholar]
- Raines D. E., Miller K. W. On the importance of volatile agents devoid of anesthetic action. Anesth Analg. 1994 Dec;79(6):1031–1033. doi: 10.1213/00000539-199412000-00001. [DOI] [PubMed] [Google Scholar]
- Smith R. A., Porter E. G., Miller K. W. The solubility of anesthetic gases in lipid bilayers. Biochim Biophys Acta. 1981 Jul 20;645(2):327–338. doi: 10.1016/0005-2736(81)90204-2. [DOI] [PubMed] [Google Scholar]
- Taheri S., Halsey M. J., Liu J., Eger E. I., 2nd, Koblin D. D., Laster M. J. What solvent best represents the site of action of inhaled anesthetics in humans, rats, and dogs? Anesth Analg. 1991 May;72(5):627–634. doi: 10.1213/00000539-199105000-00010. [DOI] [PubMed] [Google Scholar]
- Taheri S., Laster M. J., Liu J., Eger E. I., 2nd, Halsey M. J., Koblin D. D. Anesthesia by n-alkanes not consistent with the Meyer-Overton hypothesis: determinations of the solubilities of alkanes in saline and various lipids. Anesth Analg. 1993 Jul;77(1):7–11. doi: 10.1213/00000539-199307000-00003. [DOI] [PubMed] [Google Scholar]
- Trudell J. R., Hubbell W. L. Localization of molecular halothane in phospholipid bilayer model nerve membranes. Anesthesiology. 1976 Mar;44(3):202–205. doi: 10.1097/00000542-197603000-00005. [DOI] [PubMed] [Google Scholar]
- Ueda I. [Is there a receptor for volatile anesthetics?]. Masui. 1995 Apr;44(4):480–488. [PubMed] [Google Scholar]
- Wood S. C., Tonner P. H., de Armendi A. J., Bugge B., Miller K. W. Channel inhibition by alkanols occurs at a binding site on the nicotinic acetylcholine receptor. Mol Pharmacol. 1995 Jan;47(1):121–130. [PubMed] [Google Scholar]
- Xu Y., Tang P. Amphiphilic sites for general anesthetic action? Evidence from 129Xe-[1H] intermolecular nuclear Overhauser effects. Biochim Biophys Acta. 1997 Jan 14;1323(1):154–162. doi: 10.1016/s0005-2736(96)00184-8. [DOI] [PubMed] [Google Scholar]
- Xu Y., Tang P., Firestone L., Zhang T. T. 19F nuclear magnetic resonance investigation of stereoselective binding of isoflurane to bovine serum albumin. Biophys J. 1996 Jan;70(1):532–538. doi: 10.1016/S0006-3495(96)79599-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokono S., Ogli K., Miura S., Ueda I. 400 MHz two-dimensional nuclear Overhauser spectroscopy on anesthetic interaction with lipid bilayer. Biochim Biophys Acta. 1989 Jul 10;982(2):300–302. doi: 10.1016/0005-2736(89)90068-0. [DOI] [PubMed] [Google Scholar]
- Yokono S., Shieh D. D., Ueda I. Interfacial preference of anesthetic action upon the phase transition of phospholipid bilayers and partition equilibrium of inhalation anesthetics between membrane and deuterium oxide. Biochim Biophys Acta. 1981 Jul 20;645(2):237–242. doi: 10.1016/0005-2736(81)90194-2. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Takahashi K., Ueda I. Molecular orientation of volatile anesthetics at the binding surface: 1H- and 19F-NMR studies of submolecular affinity. Biochim Biophys Acta. 1989 Nov 3;985(3):331–333. doi: 10.1016/0005-2736(89)90421-5. [DOI] [PubMed] [Google Scholar]


