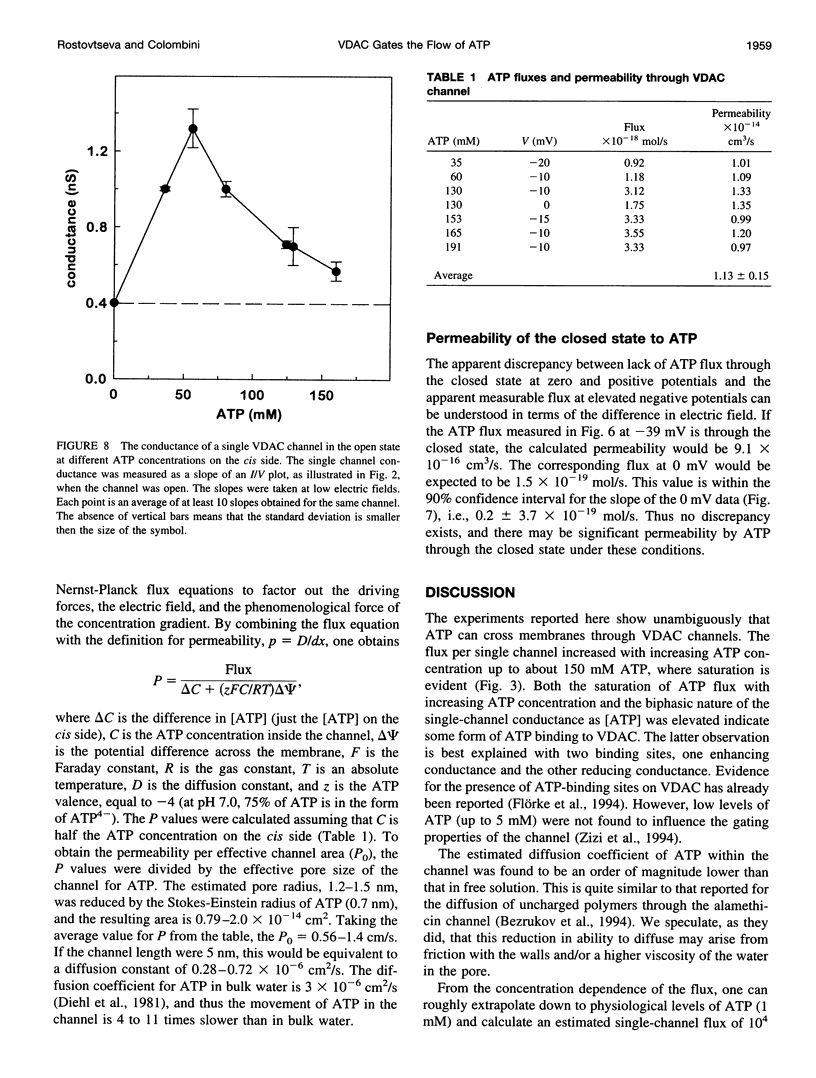
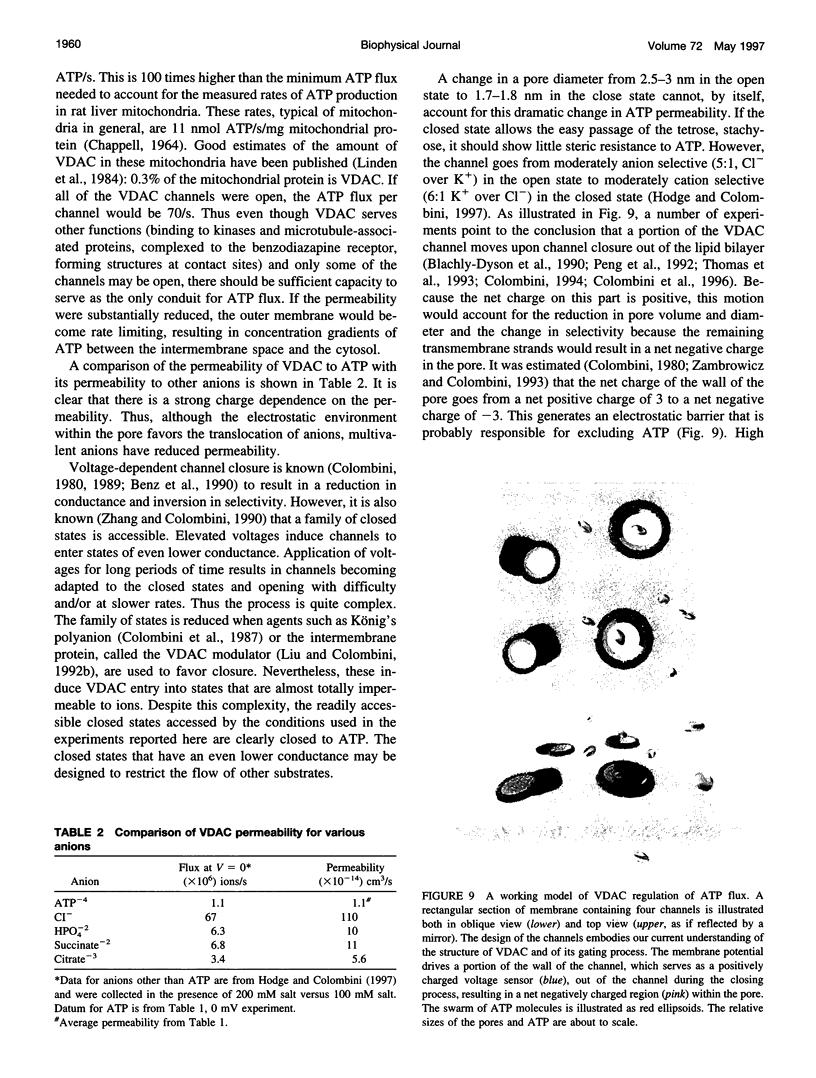
Abstract
The mitochondrial channel, VDAC, forms large (3 nm in diameter) aqueous pores through membranes. We measured ATP flow (using the luciferin/luciferase method) through these channels after reconstitution into planar phospholipid membranes. In the open state of VDAC, as many as 2 x 10(6) ATP molecules can flow through one channel per second. The half-maximum rate occurs at approximately 75 mM ATP. The permeability of a single channel for ATP is 1.1 x 10(-14) cm3/s (about 1 cm/s after correcting for cross-sectional area), which is 100 times less than the permeability for chloride and 10 times less than that for succinate. Channel closure results in a 50% reduction in conductance, showing that monovalent ions are still quite permeable, yet ATP flux is almost totally blocked. This is consistent with an electrostatic barrier that results in inversion of the selectivity of the channel and could be an example of how large channels selectively control the flow of charged metabolites. Thus VDAC is ideally suited to controlling the flow of ATP between the cytosol and the mitochondrial spaces.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benz R., Brdiczka D. The cation-selective substate of the mitochondrial outer membrane pore: single-channel conductance and influence on intermembrane and peripheral kinases. J Bioenerg Biomembr. 1992 Feb;24(1):33–39. doi: 10.1007/BF00769528. [DOI] [PubMed] [Google Scholar]
- Benz R., Kottke M., Brdiczka D. The cationically selective state of the mitochondrial outer membrane pore: a study with intact mitochondria and reconstituted mitochondrial porin. Biochim Biophys Acta. 1990 Mar;1022(3):311–318. doi: 10.1016/0005-2736(90)90279-w. [DOI] [PubMed] [Google Scholar]
- Benz R., Wojtczak L., Bosch W., Brdiczka D. Inhibition of adenine nucleotide transport through the mitochondrial porin by a synthetic polyanion. FEBS Lett. 1988 Apr 11;231(1):75–80. doi: 10.1016/0014-5793(88)80706-3. [DOI] [PubMed] [Google Scholar]
- Bezrukov S. M., Vodyanoy I., Parsegian V. A. Counting polymers moving through a single ion channel. Nature. 1994 Jul 28;370(6487):279–281. doi: 10.1038/370279a0. [DOI] [PubMed] [Google Scholar]
- Blachly-Dyson E., Peng S., Colombini M., Forte M. Selectivity changes in site-directed mutants of the VDAC ion channel: structural implications. Science. 1990 Mar 9;247(4947):1233–1236. doi: 10.1126/science.1690454. [DOI] [PubMed] [Google Scholar]
- Brdiczka D., Kaldis P., Wallimann T. In vitro complex formation between the octamer of mitochondrial creatine kinase and porin. J Biol Chem. 1994 Nov 4;269(44):27640–27644. [PubMed] [Google Scholar]
- Báthori G., Sahin-Tóth M., Fonyó A., Ligeti E. Transport properties and inhibitor sensitivity of isolated and reconstituted porin differ from those of intact mitochondria. Biochim Biophys Acta. 1993 Jan 18;1145(1):168–176. doi: 10.1016/0005-2736(93)90394-f. [DOI] [PubMed] [Google Scholar]
- Chappell J. B. The effects of 2,4-dinitrophenol on mitochondrial oxidations. Biochem J. 1964 Feb;90(2):237–248. doi: 10.1042/bj0900237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombini M., Blachly-Dyson E., Forte M. VDAC, a channel in the outer mitochondrial membrane. Ion Channels. 1996;4:169–202. doi: 10.1007/978-1-4899-1775-1_5. [DOI] [PubMed] [Google Scholar]
- Colombini M. Structure and mode of action of a voltage dependent anion-selective channel (VDAC) located in the outer mitochondrial membrane. Ann N Y Acad Sci. 1980;341:552–563. doi: 10.1111/j.1749-6632.1980.tb47198.x. [DOI] [PubMed] [Google Scholar]
- Colombini M. Voltage gating in the mitochondrial channel, VDAC. J Membr Biol. 1989 Oct;111(2):103–111. doi: 10.1007/BF01871775. [DOI] [PubMed] [Google Scholar]
- Colombini M., Yeung C. L., Tung J., König T. The mitochondrial outer membrane channel, VDAC, is regulated by a synthetic polyanion. Biochim Biophys Acta. 1987 Dec 11;905(2):279–286. doi: 10.1016/0005-2736(87)90456-1. [DOI] [PubMed] [Google Scholar]
- Fein A., Tsacopoulos M. Activation of mitochondrial oxidative metabolism by calcium ions in Limulus ventral photoreceptor. Nature. 1988 Feb 4;331(6155):437–440. doi: 10.1038/331437a0. [DOI] [PubMed] [Google Scholar]
- Flörke H., Thinnes F. P., Winkelbach H., Stadtmüller U., Paetzold G., Morys-Wortmann C., Hesse D., Sternbach H., Zimmermann B., Kaufmann-Kolle P. Channel active mammalian porin, purified from crude membrane fractions of human B lymphocytes and bovine skeletal muscle, reversibly binds adenosine triphosphate (ATP). Biol Chem Hoppe Seyler. 1994 Aug;375(8):513–520. doi: 10.1515/bchm3.1994.375.8.513. [DOI] [PubMed] [Google Scholar]
- Freitag H., Benz R., Neupert W. Isolation and properties of the porin of the outer mitochondrial membrane from Neurospora crassa. Methods Enzymol. 1983;97:286–294. doi: 10.1016/0076-6879(83)97140-9. [DOI] [PubMed] [Google Scholar]
- Gellerich F. N., Wagner M., Kapischke M., Wicker U., Brdiczka D. Effect of macromolecules on the regulation of the mitochondrial outer membrane pore and the activity of adenylate kinase in the inter-membrane space. Biochim Biophys Acta. 1993 May 6;1142(3):217–227. doi: 10.1016/0005-2728(93)90150-e. [DOI] [PubMed] [Google Scholar]
- Hajnóczky G., Robb-Gaspers L. D., Seitz M. B., Thomas A. P. Decoding of cytosolic calcium oscillations in the mitochondria. Cell. 1995 Aug 11;82(3):415–424. doi: 10.1016/0092-8674(95)90430-1. [DOI] [PubMed] [Google Scholar]
- Holden M. J., Colombini M. The mitochondrial outer membrane channel, VDAC, is modulated by a soluble protein. FEBS Lett. 1988 Dec 5;241(1-2):105–109. doi: 10.1016/0014-5793(88)81040-8. [DOI] [PubMed] [Google Scholar]
- Holden M. J., Colombini M. The outer mitochondrial membrane channel, VDAC, is modulated by a protein localized in the intermembrane space. Biochim Biophys Acta. 1993 Oct 4;1144(3):396–402. doi: 10.1016/0005-2728(93)90126-z. [DOI] [PubMed] [Google Scholar]
- Lee A. C., Zizi M., Colombini M. Beta-NADH decreases the permeability of the mitochondrial outer membrane to ADP by a factor of 6. J Biol Chem. 1994 Dec 9;269(49):30974–30980. [PubMed] [Google Scholar]
- Lindén M., Andersson G., Gellerfors P., Nelson B. D. Subcellular distribution of rat liver porin. Biochim Biophys Acta. 1984 Feb 29;770(1):93–96. doi: 10.1016/0005-2736(84)90077-4. [DOI] [PubMed] [Google Scholar]
- Liu M. Y., Colombini M. A soluble mitochondrial protein increases the voltage dependence of the mitochondrial channel, VDAC. J Bioenerg Biomembr. 1992 Feb;24(1):41–46. doi: 10.1007/BF00769529. [DOI] [PubMed] [Google Scholar]
- Liu M. Y., Colombini M. Regulation of mitochondrial respiration by controlling the permeability of the outer membrane through the mitochondrial channel, VDAC. Biochim Biophys Acta. 1992 Jan 16;1098(2):255–260. doi: 10.1016/s0005-2728(05)80344-5. [DOI] [PubMed] [Google Scholar]
- Mangan P. S., Colombini M. Ultrasteep voltage dependence in a membrane channel. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4896–4900. doi: 10.1073/pnas.84.14.4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannella C. A., Guo X. W. Interaction between the VDAC channel and a polyanionic effector. An electron microscopic study. Biophys J. 1990 Jan;57(1):23–31. doi: 10.1016/S0006-3495(90)82503-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannella C. A. Structure of the outer mitochondrial membrane: ordered arrays of porelike subunits in outer-membrane fractions from Neurospora crassa mitochondria. J Cell Biol. 1982 Sep;94(3):680–687. doi: 10.1083/jcb.94.3.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montal M., Mueller P. Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3561–3566. doi: 10.1073/pnas.69.12.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S., Blachly-Dyson E., Forte M., Colombini M. Large scale rearrangement of protein domains is associated with voltage gating of the VDAC channel. Biophys J. 1992 Apr;62(1):123–135. doi: 10.1016/S0006-3495(92)81799-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostovtseva T., Colombini M. ATP flux is controlled by a voltage-gated channel from the mitochondrial outer membrane. J Biol Chem. 1996 Nov 8;271(45):28006–28008. doi: 10.1074/jbc.271.45.28006. [DOI] [PubMed] [Google Scholar]
- Schein S. J., Colombini M., Finkelstein A. Reconstitution in planar lipid bilayers of a voltage-dependent anion-selective channel obtained from paramecium mitochondria. J Membr Biol. 1976 Dec 28;30(2):99–120. doi: 10.1007/BF01869662. [DOI] [PubMed] [Google Scholar]
- Sparagna G. C., Gunter K. K., Sheu S. S., Gunter T. E. Mitochondrial calcium uptake from physiological-type pulses of calcium. A description of the rapid uptake mode. J Biol Chem. 1995 Nov 17;270(46):27510–27515. doi: 10.1074/jbc.270.46.27510. [DOI] [PubMed] [Google Scholar]
- Thomas L., Blachly-Dyson E., Colombini M., Forte M. Mapping of residues forming the voltage sensor of the voltage-dependent anion-selective channel. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5446–5449. doi: 10.1073/pnas.90.12.5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Q., Vélez P., Brodwick M., Fill M. Streaming potentials reveal a short ryanodine-sensitive selectivity filter in cardiac Ca2+ release channel. Biophys J. 1994 Dec;67(6):2280–2285. doi: 10.1016/S0006-3495(94)80713-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambrowicz E. B., Colombini M. Zero-current potentials in a large membrane channel: a simple theory accounts for complex behavior. Biophys J. 1993 Sep;65(3):1093–1100. doi: 10.1016/S0006-3495(93)81148-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D. W., Colombini M. Group IIIA-metal hydroxides indirectly neutralize the voltage sensor of the voltage-dependent mitochondrial channel, VDAC, by interacting with a dynamic binding site. Biochim Biophys Acta. 1990 Jun 27;1025(2):127–134. doi: 10.1016/0005-2736(90)90089-7. [DOI] [PubMed] [Google Scholar]
- Zimmerberg J., Parsegian V. A. Polymer inaccessible volume changes during opening and closing of a voltage-dependent ionic channel. Nature. 1986 Sep 4;323(6083):36–39. doi: 10.1038/323036a0. [DOI] [PubMed] [Google Scholar]
- Zizi M., Forte M., Blachly-Dyson E., Colombini M. NADH regulates the gating of VDAC, the mitochondrial outer membrane channel. J Biol Chem. 1994 Jan 21;269(3):1614–1616. [PubMed] [Google Scholar]