Abstract
Previous biophysical models of ameboid crawling have described cell movement in terms of a persistent random walk. Speed and orientation were treated in the latter model as independent and temporally homogeneous stochastic processes. We show here that, at least in the case of Dictyostelium discoideum, both speed control and reorientation processes involve a deterministic, periodic component. We also show that the processes are synchronized and negatively correlated, as was suggested by earlier findings. That is, increased turning correlates with periods of slow movement. Therefore, previous models are inconsistent with the behavior of cells. Using a heuristic approach, we have developed a mathematical model that describes the statistical properties of the cell's velocity and movement of its centroid. Our observations and the model are consistent with the phenomenological description of ameboid motility as a cyclic process of pseudopod extension and retraction.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DiMilla P. A., Barbee K., Lauffenburger D. A. Mathematical model for the effects of adhesion and mechanics on cell migration speed. Biophys J. 1991 Jul;60(1):15–37. doi: 10.1016/S0006-3495(91)82027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn G. A., Brown A. F. A unified approach to analysing cell motility. J Cell Sci Suppl. 1987;8:81–102. doi: 10.1242/jcs.1987.supplement_8.5. [DOI] [PubMed] [Google Scholar]
- Franke K., Gruler H. Galvanotaxis of human granulocytes: electric field jump studies. Eur Biophys J. 1990;18(6):335–346. doi: 10.1007/BF00196924. [DOI] [PubMed] [Google Scholar]
- Gail M. H., Boone C. W. The locomotion of mouse fibroblasts in tissue culture. Biophys J. 1970 Oct;10(10):980–993. doi: 10.1016/S0006-3495(70)86347-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruler H., Bültmann B. D. Analysis of cell movement. Blood Cells. 1984;10(1):61–77. [PubMed] [Google Scholar]
- Gruler H., Franke K. Automatic control and directed cell movement. Novel approach for understanding chemotaxis, galvanotaxis, galvanotropism. Z Naturforsch C. 1990 Nov-Dec;45(11-12):1241–1249. doi: 10.1515/znc-1990-11-1226. [DOI] [PubMed] [Google Scholar]
- Gruler H., de Boisfleury Chevance A. Chemokinesis and necrotaxis of human granulocytes: the important cellular organelles. Z Naturforsch C. 1987 Sep-Oct;42(9-10):1126–1134. doi: 10.1515/znc-1987-9-1022. [DOI] [PubMed] [Google Scholar]
- Hartman R. S., Lau K., Chou W., Coates T. D. The fundamental motor of the human neutrophil is not random: evidence for local non-Markov movement in neutrophils. Biophys J. 1994 Dec;67(6):2535–2545. doi: 10.1016/S0006-3495(94)80743-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killich T., Plath P. J., Wei X., Bultmann H., Rensing L., Vicker M. G. The locomotion, shape and pseudopodial dynamics of unstimulated Dictyostelium cells are not random. J Cell Sci. 1993 Dec;106(Pt 4):1005–1013. doi: 10.1242/jcs.106.4.1005. [DOI] [PubMed] [Google Scholar]
- Lauffenburger D. A. Models for receptor-mediated cell phenomena: adhesion and migration. Annu Rev Biophys Biophys Chem. 1991;20:387–414. doi: 10.1146/annurev.bb.20.060191.002131. [DOI] [PubMed] [Google Scholar]
- Mandeville J. T., Ghosh R. N., Maxfield F. R. Intracellular calcium levels correlate with speed and persistent forward motion in migrating neutrophils. Biophys J. 1995 Apr;68(4):1207–1217. doi: 10.1016/S0006-3495(95)80336-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J., Vawter-Hugart H., Voss E., Soll D. R. Three-dimensional motility cycle in leukocytes. Cell Motil Cytoskeleton. 1992;22(3):211–223. doi: 10.1002/cm.970220308. [DOI] [PubMed] [Google Scholar]
- Nossal R., Zigmond S. H. Chemotropism indices for polymorphonuclear leukocytes. Biophys J. 1976 Oct;16(10):1171–1182. doi: 10.1016/S0006-3495(76)85766-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oster G. F., Odell G. M. Mechanics of cytogels I: oscillations in physarum. Cell Motil. 1984;4(6):469–503. doi: 10.1002/cm.970040606. [DOI] [PubMed] [Google Scholar]
- Satoh H., Ueda T., Kobatake Y. Oscillations in cell shape and size during locomotion and in contractile activities of Physarum polycephalum, Dictyostelium discoideum, Amoeba proteus and macrophages. Exp Cell Res. 1985 Jan;156(1):79–90. doi: 10.1016/0014-4827(85)90263-0. [DOI] [PubMed] [Google Scholar]
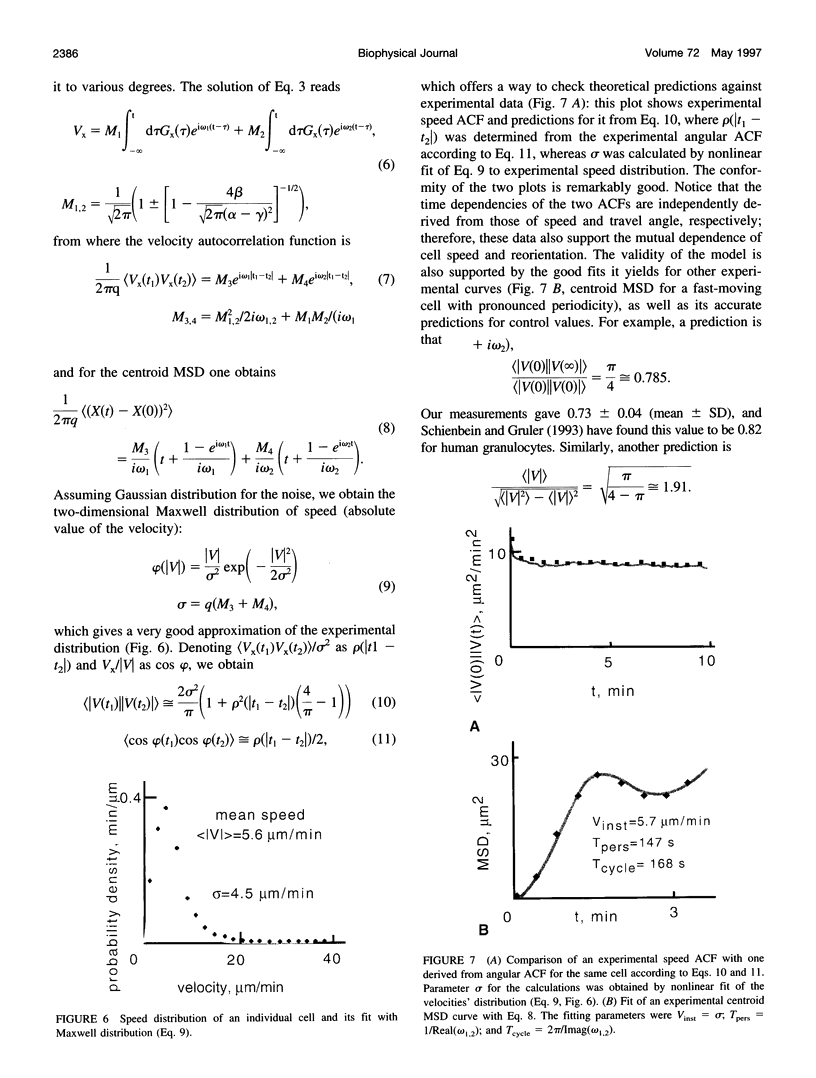
- Schienbein M., Gruler H. Langevin equation, Fokker-Planck equation and cell migration. Bull Math Biol. 1993 May;55(3):585–608. doi: 10.1007/BF02460652. [DOI] [PubMed] [Google Scholar]
- Stokes C. L., Lauffenburger D. A., Williams S. K. Migration of individual microvessel endothelial cells: stochastic model and parameter measurement. J Cell Sci. 1991 Jun;99(Pt 2):419–430. doi: 10.1242/jcs.99.2.419. [DOI] [PubMed] [Google Scholar]
- Sylwester A., Shutt D., Wessels D., Stapleton J. T., Stites J., Kennedy R. C., Soll D. R. T cells and HIV-induced T cell syncytia exhibit the same motility cycle. J Leukoc Biol. 1995 Apr;57(4):643–650. doi: 10.1002/jlb.57.4.643. [DOI] [PubMed] [Google Scholar]
- Van Duijn B., Van Haastert P. J. Independent control of locomotion and orientation during Dictyostelium discoideum chemotaxis. J Cell Sci. 1992 Aug;102(Pt 4):763–768. doi: 10.1242/jcs.102.4.763. [DOI] [PubMed] [Google Scholar]
- Wessels D., Vawter-Hugart H., Murray J., Soll D. R. Three-dimensional dynamics of pseudopod formation and the regulation of turning during the motility cycle of Dictyostelium. Cell Motil Cytoskeleton. 1994;27(1):1–12. doi: 10.1002/cm.970270102. [DOI] [PubMed] [Google Scholar]