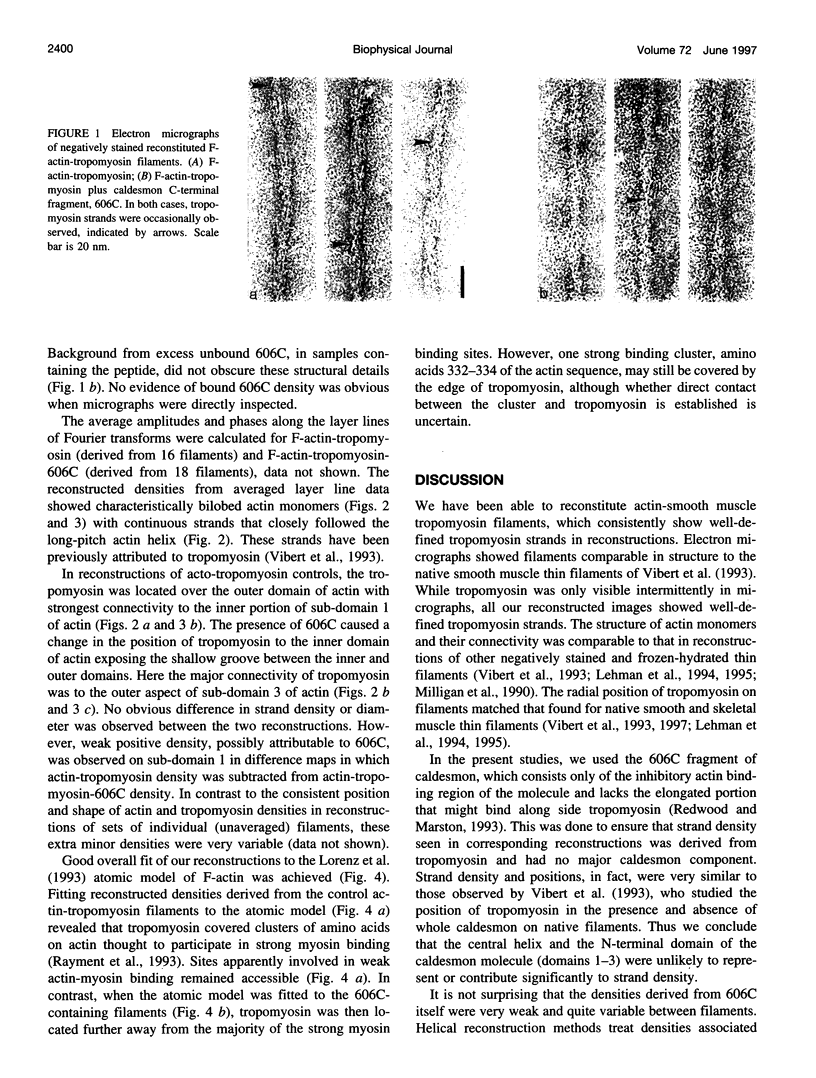
Abstract
Caldesmon inhibits actomyosin ATPase and filament sliding in vitro, and therefore may play a role in modulating smooth and non-muscle motile activities. A bacterially expressed caldesmon fragment, 606C, which consists of the C-terminal 150 amino acids of the intact molecule, possesses the same inhibitory properties as full-length caldesmon and was used in our structural studies to examine caldesmon function. Three-dimensional image reconstruction was carried out from electron micrographs of negatively stained, reconstituted thin filaments consisting of actin and smooth muscle tropomyosin both with and without added 606C. Helically arranged actin monomers and tropomyosin strands were observed in both cases. In the absence of 606C, tropomyosin adopted a position on the inner edge of the outer domain of actin monomers, with an apparent connection to sub-domain 1 of actin. In 606C-containing filaments that inhibited acto-HMM ATPase activity, tropomyosin was found in a different position, in association with the inner domain of actin, away from the majority of strong myosin binding sites. The effect of caldesmon on tropomyosin position therefore differs from that of troponin on skeletal muscle filaments, implying that caldesmon and troponin act by different structural mechanisms.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amos L. A., Klug A. Three-dimensional image reconstructions of the contractile tail of T4 bacteriophage. J Mol Biol. 1975 Nov 25;99(1):51–64. doi: 10.1016/s0022-2836(75)80158-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey K. Tropomyosin: a new asymmetric protein component of the muscle fibril. Biochem J. 1948;43(2):271–279. doi: 10.1042/bj0430271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacko S., Eisenberg E. Cooperativity of actin-activated ATPase of gizzard heavy meromyosin in the presence of gizzard tropomyosin. J Biol Chem. 1990 Feb 5;265(4):2105–2110. [PubMed] [Google Scholar]
- Chalovich J. M. Actin mediated regulation of muscle contraction. Pharmacol Ther. 1992;55(2):95–148. doi: 10.1016/0163-7258(92)90013-p. [DOI] [PubMed] [Google Scholar]
- Dabrowska R., Goch A., Gałazkiewicz B., Osińska H. The influence of caldesmon on ATPase activity of the skeletal muscle actomyosin and bundling of actin filaments. Biochim Biophys Acta. 1985 Sep 27;842(1):70–75. doi: 10.1016/0304-4165(85)90295-8. [DOI] [PubMed] [Google Scholar]
- DeRosier D. J., Moore P. B. Reconstruction of three-dimensional images from electron micrographs of structures with helical symmetry. J Mol Biol. 1970 Sep 14;52(2):355–369. doi: 10.1016/0022-2836(70)90036-7. [DOI] [PubMed] [Google Scholar]
- Egelman E. H. An algorithm for straightening images of curved filamentous structures. Ultramicroscopy. 1986;19(4):367–373. doi: 10.1016/0304-3991(86)90096-3. [DOI] [PubMed] [Google Scholar]
- Eisenberg E., Kielley W. W. Troponin-tropomyosin complex. Column chromatographic separation and activity of the three, active troponin components with and without tropomyosin present. J Biol Chem. 1974 Aug 10;249(15):4742–4748. [PubMed] [Google Scholar]
- Fraser I. D., Marston S. B. In vitro motility analysis of smooth muscle caldesmon control of actin-tropomyosin filament movement. J Biol Chem. 1995 Aug 25;270(34):19688–19693. doi: 10.1074/jbc.270.34.19688. [DOI] [PubMed] [Google Scholar]
- Fürst D. O., Cross R. A., De Mey J., Small J. V. Caldesmon is an elongated, flexible molecule localized in the actomyosin domains of smooth muscle. EMBO J. 1986 Feb;5(2):251–257. doi: 10.1002/j.1460-2075.1986.tb04206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geeves M. A., Halsall D. J. Two-step ligand binding and cooperativity. A model to describe the cooperative binding of myosin subfragment 1 to regulated actin. Biophys J. 1987 Aug;52(2):215–220. doi: 10.1016/S0006-3495(87)83208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeberle J. R., Trybus K. M., Hemric M. E., Warshaw D. M. The effects of smooth muscle caldesmon on actin filament motility. J Biol Chem. 1992 Nov 15;267(32):23001–23006. [PubMed] [Google Scholar]
- Hill T. L., Eisenberg E., Greene L. Theoretical model for the cooperative equilibrium binding of myosin subfragment 1 to the actin-troponin-tropomyosin complex. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3186–3190. doi: 10.1073/pnas.77.6.3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi K. Y., Chacko S. Caldesmon inhibits the cooperative turning-on of the smooth muscle heavy meromyosin by tropomyosin-actin. Biochemistry. 1989 Nov 14;28(23):9111–9116. doi: 10.1021/bi00449a023. [DOI] [PubMed] [Google Scholar]
- Jones T. A., Zou J. Y., Cowan S. W., Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A. 1991 Mar 1;47(Pt 2):110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- Kamm K. E., Stull J. T. The function of myosin and myosin light chain kinase phosphorylation in smooth muscle. Annu Rev Pharmacol Toxicol. 1985;25:593–620. doi: 10.1146/annurev.pa.25.040185.003113. [DOI] [PubMed] [Google Scholar]
- Lehman W., Craig R., Lui J., Moody C. Caldesmon and the structure of smooth muscle thin filaments: immunolocalization of caldesmon on thin filaments. J Muscle Res Cell Motil. 1989 Apr;10(2):101–112. doi: 10.1007/BF01739966. [DOI] [PubMed] [Google Scholar]
- Lehman W., Craig R., Vibert P. Ca(2+)-induced tropomyosin movement in Limulus thin filaments revealed by three-dimensional reconstruction. Nature. 1994 Mar 3;368(6466):65–67. doi: 10.1038/368065a0. [DOI] [PubMed] [Google Scholar]
- Lehman W., Vibert P., Uman P., Craig R. Steric-blocking by tropomyosin visualized in relaxed vertebrate muscle thin filaments. J Mol Biol. 1995 Aug 11;251(2):191–196. doi: 10.1006/jmbi.1995.0425. [DOI] [PubMed] [Google Scholar]
- Lehrer S. S. The regulatory switch of the muscle thin filament: Ca2+ or myosin heads? J Muscle Res Cell Motil. 1994 Jun;15(3):232–236. doi: 10.1007/BF00123476. [DOI] [PubMed] [Google Scholar]
- Levine B. A., Moir A. J., Audemard E., Mornet D., Patchell V. B., Perry S. V. Structural study of gizzard caldesmon and its interaction with actin. Binding involves residues of actin also recognised by myosin subfragment 1. Eur J Biochem. 1990 Nov 13;193(3):687–696. doi: 10.1111/j.1432-1033.1990.tb19388.x. [DOI] [PubMed] [Google Scholar]
- Lorenz M., Popp D., Holmes K. C. Refinement of the F-actin model against X-ray fiber diffraction data by the use of a directed mutation algorithm. J Mol Biol. 1993 Dec 5;234(3):826–836. doi: 10.1006/jmbi.1993.1628. [DOI] [PubMed] [Google Scholar]
- Mabuchi K., Li Y., Tao T., Wang C. L. Immunocytochemical localization of caldesmon and calponin in chicken gizzard smooth muscle. J Muscle Res Cell Motil. 1996 Apr;17(2):243–260. doi: 10.1007/BF00124246. [DOI] [PubMed] [Google Scholar]
- Margossian S. S., Lowey S. Preparation of myosin and its subfragments from rabbit skeletal muscle. Methods Enzymol. 1982;85(Pt B):55–71. doi: 10.1016/0076-6879(82)85009-x. [DOI] [PubMed] [Google Scholar]
- Marston S. B., Fraser I. D., Huber P. A. Smooth muscle caldesmon controls the strong binding interaction between actin-tropomyosin and myosin. J Biol Chem. 1994 Dec 23;269(51):32104–32109. [PubMed] [Google Scholar]
- Marston S. B., Redwood C. S. Inhibition of actin-tropomyosin activation of myosin MgATPase activity by the smooth muscle regulatory protein caldesmon. J Biol Chem. 1992 Aug 25;267(24):16796–16800. [PubMed] [Google Scholar]
- Marston S. B., Redwood C. S. The essential role of tropomyosin in cooperative regulation of smooth muscle thin filament activity by caldesmon. J Biol Chem. 1993 Jun 15;268(17):12317–12320. [PubMed] [Google Scholar]
- Marston S. B., Smith C. W. The thin filaments of smooth muscles. J Muscle Res Cell Motil. 1985 Dec;6(6):669–708. doi: 10.1007/BF00712237. [DOI] [PubMed] [Google Scholar]
- Marston S. Stoichiometry and stability of caldesmon in native thin filaments from sheep aorta smooth muscle. Biochem J. 1990 Dec 1;272(2):305–310. doi: 10.1042/bj2720305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGough A., Way M. Molecular model of an actin filament capped by a severing protein. J Struct Biol. 1995 Sep-Oct;115(2):144–150. doi: 10.1006/jsbi.1995.1038. [DOI] [PubMed] [Google Scholar]
- McLachlan A. D., Stewart M. The 14-fold periodicity in alpha-tropomyosin and the interaction with actin. J Mol Biol. 1976 May 15;103(2):271–298. doi: 10.1016/0022-2836(76)90313-2. [DOI] [PubMed] [Google Scholar]
- Milligan R. A., Flicker P. F. Structural relationships of actin, myosin, and tropomyosin revealed by cryo-electron microscopy. J Cell Biol. 1987 Jul;105(1):29–39. doi: 10.1083/jcb.105.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan R. A., Whittaker M., Safer D. Molecular structure of F-actin and location of surface binding sites. Nature. 1990 Nov 15;348(6298):217–221. doi: 10.1038/348217a0. [DOI] [PubMed] [Google Scholar]
- Moody C., Lehman W., Craig R. Caldesmon and the structure of smooth muscle thin filaments: electron microscopy of isolated thin filaments. J Muscle Res Cell Motil. 1990 Apr;11(2):176–185. doi: 10.1007/BF01766496. [DOI] [PubMed] [Google Scholar]
- Mornet D., Bonet-Kerrache A., Strasburg G. M., Patchell V. B., Perry S. V., Huber P. A., Marston S. B., Slatter D. A., Evans J. S., Levine B. A. The binding of distinct segments of actin to multiple sites in the C-terminus of caldesmon: comparative aspects of actin interaction with troponin-I and caldesmon. Biochemistry. 1995 Feb 14;34(6):1893–1901. doi: 10.1021/bi00006a010. [DOI] [PubMed] [Google Scholar]
- Ngai P. K., Walsh M. P. Inhibition of smooth muscle actin-activated myosin Mg2+-ATPase activity by caldesmon. J Biol Chem. 1984 Nov 25;259(22):13656–13659. [PubMed] [Google Scholar]
- North A. J., Gimona M., Cross R. A., Small J. V. Calponin is localised in both the contractile apparatus and the cytoskeleton of smooth muscle cells. J Cell Sci. 1994 Mar;107(Pt 3):437–444. doi: 10.1242/jcs.107.3.437. [DOI] [PubMed] [Google Scholar]
- Okagaki T., Higashi-Fujime S., Ishikawa R., Takano-Ohmuro H., Kohama K. In vitro movement of actin filaments on gizzard smooth muscle myosin: requirement of phosphorylation of myosin light chain and effects of tropomyosin and caldesmon. J Biochem. 1991 Jun;109(6):858–866. doi: 10.1093/oxfordjournals.jbchem.a123471. [DOI] [PubMed] [Google Scholar]
- Phillips G. N., Jr, Fillers J. P., Cohen C. Tropomyosin crystal structure and muscle regulation. J Mol Biol. 1986 Nov 5;192(1):111–131. doi: 10.1016/0022-2836(86)90468-7. [DOI] [PubMed] [Google Scholar]
- Rayment I., Holden H. M., Whittaker M., Yohn C. B., Lorenz M., Holmes K. C., Milligan R. A. Structure of the actin-myosin complex and its implications for muscle contraction. Science. 1993 Jul 2;261(5117):58–65. doi: 10.1126/science.8316858. [DOI] [PubMed] [Google Scholar]
- Redwood C. S., Marston S. B. Binding and regulatory properties of expressed functional domains of chicken gizzard smooth muscle caldesmon. J Biol Chem. 1993 May 25;268(15):10969–10976. [PubMed] [Google Scholar]
- Sanders C., Smillie L. B. Chicken gizzard tropomyosin: head-to-tail assembly and interaction with F-actin and troponin. Can J Biochem Cell Biol. 1984 Jun;62(6):443–448. doi: 10.1139/o84-060. [DOI] [PubMed] [Google Scholar]
- Shirinsky V. P., Biryukov K. G., Hettasch J. M., Sellers J. R. Inhibition of the relative movement of actin and myosin by caldesmon and calponin. J Biol Chem. 1992 Aug 5;267(22):15886–15892. [PubMed] [Google Scholar]
- Smith C. W., Pritchard K., Marston S. B. The mechanism of Ca2+ regulation of vascular smooth muscle thin filaments by caldesmon and calmodulin. J Biol Chem. 1987 Jan 5;262(1):116–122. [PubMed] [Google Scholar]
- Szpacenko A., Dabrowska R. Functional domain of caldesmon. FEBS Lett. 1986 Jul 7;202(2):182–186. doi: 10.1016/0014-5793(86)80683-4. [DOI] [PubMed] [Google Scholar]
- Trachtenberg S., DeRosier D. J. Three-dimensional structure of the frozen-hydrated flagellar filament. The left-handed filament of Salmonella typhimurium. J Mol Biol. 1987 Jun 5;195(3):581–601. doi: 10.1016/0022-2836(87)90184-7. [DOI] [PubMed] [Google Scholar]
- Vibert P., Craig R., Lehman W. Steric-model for activation of muscle thin filaments. J Mol Biol. 1997 Feb 14;266(1):8–14. doi: 10.1006/jmbi.1996.0800. [DOI] [PubMed] [Google Scholar]
- Vibert P. Helical reconstruction of frozen-hydrated scallop myosin filaments. J Mol Biol. 1992 Feb 5;223(3):661–671. doi: 10.1016/0022-2836(92)90982-p. [DOI] [PubMed] [Google Scholar]
- Williams D. L., Jr, Greene L. E., Eisenberg E. Comparison of effects of smooth and skeletal muscle tropomyosins on interactions of actin and myosin subfragment 1. Biochemistry. 1984 Aug 28;23(18):4150–4155. doi: 10.1021/bi00313a022. [DOI] [PubMed] [Google Scholar]