Abstract
BACKGROUND:
Following lower limb amputation, timely prosthetic fitting enhances mobility and quality of life. However, inconsistent definitions of surgical site healing complicate prosthesis readiness assessment and highlight the need for objective wound management measures.
OBJECTIVE:
This review aimed to compile definitions of healing and non-healing provided in the literature investigating biomarkers of healing of the tissues and structures found in the residual limbs of adults with amputation.
METHODOLOGY:
A scoping review was conducted following JBI and PRISMA-ScR guidance. Searches using “biomarkers,” “wound healing,” and “amputation” were performed on May 6, 2023, on Web of Science, Ovid MEDLINE, Ovid Embase, Scopus, Cochrane, PubMed, and CINAHL databases. Inclusion criteria were: 1) References to biomarkers and healing; 2) Residuum tissue healing; 3) Clear methodology with ethical approval; 4) Published from 2017 onwards. Articles were assessed for quality (QualSyst tool) and evidence level (JBI system).
FINDINGS:
Of 3,306 articles screened, 219 met the inclusion criteria and are reviewed in this article, with 77% rated strong quality. 43% of all included sources did not define healing, while the remainder used specific criteria including epithelialization (14%), wound size reduction (28%), gradings scales (3%), scarring (1%), absence of wound complications (2%), hydroxyproline levels (0.5%), no amputation (0.5%), or neovascularization (0.5%). 84% of included sources did not provide definitions of non-healing. Studies defining non-healing used criteria like wound complications (4%), the need for operative interventions (4%), or lack of wound size reduction (1%). For 10% of included sources, healing and non-healing definitions were considered not applicable given the research content. Total percentages exceed 100% for both healing and non-healing definitions because some sources used two definition classifications, such as epithelialization and wound size reduction. The findings indicate a lack of standardized definitions irrespective of study type.
CONCLUSION:
This review reveals significant gaps in current definitions of healing and non-healing, often based on superficial assessments that overlook deeper tissue healing and mechanical properties essential for prosthesis use. It emphasizes the need for comprehensive definitions incorporating biomarkers and psychosocial factors to improve wound management and post-amputation recovery.
Keywords: Amputation, Scoping Review, Wound Healing, Wound Non-Healing, Surgical Site Healing, Biomarkers, Markers of Healing, Residuum Healing, Residual Limb Healing, Wound Management, Impaired Healing.
INTRODUCTION
1: OVERALL RATIONALE, AIMS, AND OBJECTIVES
The term “wound” broadly refers to damage to any biological tissue,1 encompassing damage from amputation surgery to deep tissue injuries caused by loading during lower limb prosthetic use. The healthy, or normal, wound healing process is marked by four interlinked physiologic phases (Table 1): I) hemostasis, II) inflammation, III) proliferation, and IV) tissue remodeling (or resolution).2–4 This complex process demands a high degree of cellular coordination, including several avenues through which impairments can occur. Consequently, wound healing can be stalled (also referred to as non-healing, impaired, or chronic) not by one isolated factor, but by several smaller contributing issues.5 For example, common post-amputation surgical site healing complications include infection, pain, hematomas, tissue necrosis, poor residual limb formation, recurrent ulceration, wound dehiscence, and stitch abscesses.6,7 Persistent complications, in other words, poor healing, can necessitate revision surgeries or even re-amputation at more proximal levels.6 Despite the intricacies of the wound healing process, the current assessment of healing relies mainly on surface level clinician examinations and wound classification systems. For instance, the East London NHS (National Health Service) Trust's clinical guidelines recommend using a disposable measuring tape to monitor wound healing by assessing wound length and width.8 Such subjective methods introduce biases and fail to account for underlying issues. Deep tissue injuries (DTIs), for example, develop subcutaneously and only become visible in later stages, manifesting as bruised purple localized areas of intact skin9 that can evolve into large deep wounds.10 This introduces the need for more objective measures to assess healing both at the surface level and below the cutaneous layer.
Table 1*:
Characteristics and time frames of the four primary interlinked phases of wound healing.
| Phase | Characterization | Time Frame |
|---|---|---|
| I (Hemostasis) | Directly after injury, there is an outpouring of lymphatic fluid and blood. This involves platelet aggregation (blood clotting) and blood vessel vasoconstriction to prevent further bleeding. | Seconds to Hours |
| II (Inflammation) | Cellular debris and bacteria are removed. Vascular permeability is increased to promote the diffusion of necessary molecules to the wound site. Cellular migration is similarly increased, as is chemotaxis. The aim is to limit further damage. | Hours to Days |
| III (Proliferation) | Formation of granulation tissue (the contractile organ that fills wounds that heal by second intention), reepithelization (epidermis regeneration), and neovascularization. | Days to Weeks |
| IV (Remodeling) | Defined by vascular maturation and regression, and collagen remodeling. The wound reaches its maximum strength and its ultimate endpoint; in cutaneous tissue, this is marked by a collagenous scar. | Weeks to Months |
Adapted from References 2–4.
This necessity for more objective measures is particularly pertinent in managing residual limbs following lower limb amputation. Following their surgery, depending on the healing process, individuals who have undergone lower limb amputation will typically receive a customized prosthetic limb within a window of 3 to 20 weeks post-surgery.11,12 These prosthetic interventions are bespoke devices aimed to replicate the missing limb function, enhancing the user's mobility, ambulation, and ability to perform daily tasks. Consequently, they significantly improve physical health, cardiovascular well-being, mental health, quality of life, and overall independence.12,13 Notably, Singh and Prasad14 reported that the absence of a prosthetic limb fitting is an independent predictor of mortality within three years of a major lower limb amputation, defined as the loss of the limb at or proximal to the ankle joint.15
However, assessing residuum healing and thus readiness for a prosthesis after amputation, like wound healing, remains ambiguous, involving clinician opinion, and surface level wound examination. In a narrative review of determinants of healing and readiness for prosthetic fitting after transtibial amputation, Day et al.16 concluded that clinical judgement is most subjective when assessing the degree of healing. Online resources for individuals with amputation similarly note that readiness for prosthetic fitting is dependent on factors such as healing, pain management, oedema, and residual limb volume,17 yet specific indicators for these factors remain undefined. Even healthcare bodies like the NHS provide no clear guidelines on assessing readiness, relying instead on clinicians' experience and judgement, which can vary widely. For instance, Turner et al.18 in their thematic analysis of issues faced by prosthetists and physiotherapists during lower limb prosthetic rehabilitation, noted that clinicians lack a standardized approach to prosthetic rehabilitation. To illustrate, some prosthetists prefer removing a prosthesis to promote wound healing, whereas others believe continuing to wear it is more beneficial by encouraging blood flow.18 Furthermore, recent studies suggest that a limb does not need to be fully healed to begin prosthetic rehabilitation,16 but clear guidelines for when an open surgical site is appropriate for prosthetic use are still lacking. One prosthetist emphasized18 that “we have to go at the rate of the body,” noting that limbs heal and mature at different rates, further underscoring the variability in both clinical practice and patient recovery trajectories. Moreover, individuals awaiting amputation often present with multiple comorbidities that complicate their healing process. One of the most common causes of amputation is complications arising from diabetes,19 yet hyperglycemia can lead to vascular stiffening, microvascular dysfunction, reduced tissue oxygenation, and, consequently, impaired wound healing.20
The complexity of defining readiness for prosthetic rehabilitation, coupled with the lack of standardized clinical practices, suggests the need for more objective measures, such as biomarkers, to assess healing and reduce the risk of complications like revision surgeries or re-amputations. A biomarker is defined by the U.S. FDA (Food & Drug Administration) as a “defined characteristic that is measured as an indicator of normal biological processes, a pathogenic process or a response to an exposure or intervention”.21 Additional scholarly works have extended the FDA's definition by emphasizing the requirement for objectivity22 and the importance of accurate and reproducible measurements.23 However, to the authors' knowledge, research into the use of biomarkers for monitoring healing and facilitating early prosthetic rehabilitation post-amputation remains limited. Studies that do exist, such as those focusing on changes in tissue composition during prosthetic use,24 typically examine mature residual limbs, whereas early-stage residual limbs face greater risks of complications like ulceration and volume changes, which exacerbate poor socket fit.25 Research into these early stages is crucial for ensuring successful prosthetic rehabilitation and preventing further surgical interventions.
This raises the following research question: What biomarkers (physical, chemical, or other) are predictive, diagnostic, and/or indicative of healing of the tissues and structures found in the residual limbs of adults with amputation?
In summary, as noted by Patel et al.26 advances in genomics, proteomics, and molecular pathology have led to the identification of several candidate biomarkers with potential clinical value. However, progress in this area remains slow, and there is little consensus in the literature regarding the most appropriate biomarkers for assessing healing.22 Furthermore, to the authors' knowledge, no comprehensive review exists that synthesizes biomarkers specifically related to healing after amputation.
The most recent study examining readiness for prosthetic rehabilitation following transtibial amputation concluded that the only objective healing assessment used in the included studies was transcutaneous oxygen perfusion, a physical biomarker.16 The review emphasized that objective methodologies like this could quantify healing, reduce subjectivity, and promote comparative research on different enhanced recovery after surgery protocols and their effects on post-amputation healing.16
Existing reviews are typically narrative in nature, discussing general wound healing biomarkers without a systematic approach, further highlighting the need for a more structured review of biomarkers specific to healing in the context of amputation and primary wound healing post-surgery. To address this gap a scoping review was developed and implemented to compile the breadth of available wound healing biomarker evidence and answer the research question. The aim of the review was therefore to identify predictive, diagnostic, and/or indicative biomarkers (physical, chemical, or other) of healing of the tissues and structures found in the residual limbs of adults with amputation. To meet this aim and answer the research question, the following objectives were compiled:
1) Collate and synthesize the reported definitions of healing and non-healing in the literature investigating healing of the tissues and structures found in the residual limbs of adults with amputation.
2) Identify and collate physical biomarkers predictive, diagnostic, and/or indicative of healing repeated in sources investigating healing of the tissues and structures found in the residual limbs of adults with amputation.
3) Identify and collate chemical biomarkers predictive, diagnostic, and/or indicative of healing repeated in sources investigating healing of the tissues and structures found in the residual limbs of adults with amputation.
4) Assess the quality and levels of evidence of sources investigating healing of the tissues and structures found in the residual limbs of adults with amputation.
The term “physical” refers to biomarkers such as pH, temperature of the wound, or collagen quantity revealed through histochemical staining,27 whereas the term “chemical” refers to markers found in wound tissue, fluid, serum/blood, sebum, saliva, or sweat such as cytokines or matrix metalloproteinases (MMPs).
2: PART 1 - RATIONALE, AIMS, AND OBJECTIVES
This article (Part 1) addresses Objectives 1 and 4 and is the first in a series of three articles, each of which explores Objectives 1 to 3 in turn. Before objective measures of healing can be developed, it is essential to first clarify the current definitions of healing. The timing of prosthetic rehabilitation, for instance, is contingent upon how healing, and consequently readiness for prosthetic fitting, is defined. Likewise, effective wound management hinges on the criteria used to distinguish between a healed and an unhealed wound. However, the literature reveals a lack of consensus on the definitions of healing and non-healing wounds.28
While complete healing is often characterized by the “complete epithelialization” of the wound,29–31 this description neglects the underlying tissue layers. Where definitions of healing fall short, defining non-healing may be a useful alternative. Yet, definitions of impaired healing (commonly referred to as non-healing, chronic wound healing, or delayed healing) also exhibit significant variability. For instance, Furuyama et al.32 define non-healing ulcers as wounds resulting in “major amputation or death before achieving ulcer healing”, whereas another source considers a chronic wound to be one that “has not shown a 20–40% reduction in wound area after 2–4 weeks of optimal treatment”.33 Relying solely on temporal criteria to distinguish healing from non-healing can be problematic. For example, research has shown that while older adults may experience delayed healing, the ultimate outcome remains comparable to that of younger individuals.34 Additionally, Day et al.16 found that in their review of determinants of healing and readiness for prosthetic fitting, healing was undefined in 13 of the 15 studies reviewed. They also noted that the absence of standard healing definitions, the heterogeneity of measurable endpoints, and the inconsistent reporting of healing across studies significantly hinder the extrapolation of findings.
In light of these challenges, the following article aims to answer the research question: How are healing and non-healing defined in the literature investigating biomarkers of healing of the tissues and structures found in the residual limbs of adults with amputation?
The aim of this article is therefore to compile definitions of healing and non-healing that are provided in the literature investigating biomarkers of healing of tissues and structures found in the residual limbs of adults with amputation.
METHODOLOGY
Given the novelty of the research question and the variable sources available on biomarkers, a scoping review was deemed the most appropriate method to meet the aims and objectives and answer the research question. The scoping review was based upon the Joanna Briggs Institute (JBI) methodology for scoping reviews35–38 and implemented following the Preferred Reporting Items for Systematic Reviews extension for Scoping Reviews (PRISMA-ScR) checklist and guidance.39,40 All results were tracked and recorded on Excel Version Number 2303 (Microsoft, Washington, USA) run on Windows 11 Version 22H2 (Microsoft, Washington, USA). A scoping review is iterative,41 with several steps requiring piloting; thus, the methodology presented in the following sections represents the final iterations of these processes.
1: INCLUSION CRITERIA
The following sections detail and rationalize the inclusion criteria of the scoping review culminating in the generation of an inclusion tool (Table 2) used in the first and second rounds of screening.
Table 2:
Inclusion criteria tool applied to each source during the first (title and abstracts) and second (full text) screening processes. To pass screening one, sources required all ‘Yes’ or ‘Maybe’ answers. To pass screening two on the other hand, and be included in data extraction, sources needed ‘Yes’ responses to all inclusion criteria.
| Evidence Source Details and Characteristics | ||||
|---|---|---|---|---|
| Citation | ||||
| Primary Author (Year) | ||||
| Title | ||||
| Abstract | ||||
| Inclusion Criteria for Screening One | Yes | No | Maybe | |
| 1 | Does it reference biomarkers of wound healing (progression/monitoring/prediction)? | |||
| 2 | Does it refer to healing of tissues found in the residuum? | |||
| 3 | Is it published during or after 2017? | |||
| Inclusion Criteria for Screening Two | Yes | No | ||
| 1 | Does it reference biomarkers of wound healing (progression/monitoring/prediction)? | |||
| 2 | Does it refer to healing of tissues found in the residuum? | |||
| 3 | Does the source involve human/rat/mice participants? If it involves human participants, are they over 18 years old? | |||
| 4 | Is it published during or after 2017? | |||
| 5 | Is the methodology clear/repeatable? | |||
| 6 | Does the study have clear ethical approval? | |||
1.1: Participants
To minimize the ethical considerations associated with studies involving children, given that healing in adults and children reportedly differs,42,43 only sources involving adult participants were included. In line with common practice in literature44 and UK law (the setting in which this research takes place), an adult is defined as an individual older than or equal to 18 years of age.45
A further inclusionary criterion was that participants must be experiencing some form of clearly described wound in tissues and structures comparable to that of an amputation residuum (Table 3). For example, the study by Giesen et al.46 meets the inclusion criteria despite focusing on risk factors, such as C-reactive protein (CRP) biomarker levels, for surgical site infections (SSI) following appendectomy. SSI is relevant as it can result in a non-healing wound.47 Although the infection in this case occurs at the appendix, it affects the surrounding skin and soft tissue. This tissue is biologically comparable to that found at an amputation surgical site, thereby making the findings applicable to the study's context.
Table 3:
For clarity this table provides examples of tissues/structures found in the amputation residuum and those not.
| Examples of Tissues/Structures Found in the Residuum |
| Skin, muscle and tendons, ligaments, bone, vasculature, and the peripheral nervous system. |
| Examples of Tissue/Structures Not Found in the Residuum |
| The central nervous system, and organs like the heart, brain, stomach, intestines, etc. |
1.2: Types of Sources
All the source types expressed in the following list were considered for inclusion to ensure the breadth of research was captured:
-
Quantitative studies
This includes any study design, including retrospective/prospective cohort studies, randomized controlled trials (RCTs),48 and in vitro, in silico, or rat/mouse studies. Note that rats/mice are considered sufficiently genetically similar to humans and are often used in biological research49 and will thus be included in this review. Where human participants were involved, the articles must clearly state whether ethical approval and informed consent were provided to meet the eligibility criteria.
Qualitative studies
Mixed studies
Case studies
Conference proceedings
Dissertations and theses
Text and opinion articles
-
Letters to editors
These may be of value given their purpose to act as a form of post-publication peer review and the platform they give researchers to share experiences with fellow readers.50
-
Guidelines issued by national and international wound and tissue viability associations
Examples of this include the National Institute for Health and Care Excellence (NICE) guidance on “Prontosan for treating acute and chronic wounds”51 and the NHS “Wound Management Clinical Practice Guidelines”.8
However, all sources included were required to be reproducible, necessitating that their methodologies be clearly outlined. As a result, sources such as letters to editors and conference proceedings generally did not meet the inclusion criteria (Figure 1). Review articles were considered secondary sources and excluded.
Figure 1:
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram of the scoping review screening process.
The extensive number of sources generated during the initial searches prompted a reassessment of the inclusion criteria. Additionally, the rapid advancements in wound healing biomarkers48 underscored the necessity for more recent data. A recent scoping review examined prognostic factors (biomarkers) associated with ulcer healing, a common diabetic complication that can precede amputation,52 specifically focusing on sources published before 2017.53 In light of this context, it was decided to include only sources published in or after 2017, thereby ensuring the relevance and timeliness of the reviewed literature.
1.3: Concept (Interventions and Outcomes)
Sources were required to explore biomarker(s) in conjunction with wound healing. A relationship between the biomarker (independent variable) and non-healing/healing (dependent variable) was required for quantitative, observational, and mixed studies. A result was considered conclusive when a statistical significance of p < 0.05 was achieved. However, measuring biomarkers can be a continuous or categorical variable, thus any sources using cut-off or dichotomizing/categorizing approaches were also included.48
1.4: Context
Sources of any context (e.g., home, hospital, community, or academic institutions) and from any discipline (e.g., healthcare professionals or engineers) were considered to capture as much research as possible. Similarly, provided they were in the English language due to the linguistic limitations of the primary reviewer, sources from any geographical setting were considered to minimize high-income-country (HIC) and Western publication bias.54,55
2: SEARCH METHODS FOR IDENTIFICATION OF SOURCES
According to the three-step search strategy recommended by JBI, an initial search was carried out on Medline via Ovid and PubMed to locate relevant sources and determine whether or not they could contribute to increasing search terms and keywords.56 Following the generation of an exhaustive list of terms based on the research question, and search strategy piloting, the search terms detailed in Table 4 were decided upon.
Table 4:
Search terms and indexing used to generate all sources screened in the final scoping review. Note the proximity search “adj5” index applies only to Ovid databases and differs according to the database.
| Biomarker | Biomarker* |
| Marker* | |
| Indicator* | |
| Factor* | |
| Amputation | Amputee* |
| Amputation* | |
| Residuum* | |
| Stump* | |
| Limb Loss | |
| Wound Healing | Wound adj5 Sensing |
| Wound adj5 Sensor | |
| Heal/Heals/Healed/Healing | |
| Monitor/Monitoring | |
| Sensor/Sense/Sensing | |
| Wound adj5 Healing | |
| Wound adj5 Monitoring | |
| Wound adj5 Monitor |
In a scoping review of scoping reviews, Pham et al.57 concluded that the most frequent limitation was the possibility of missing relevant sources, which can be attributed to database selection. To counteract this, a significant number of databases mentioned in previous scoping reviews of a similar nature48,58,59 were searched:
Web of Science
MELDINE (hosted on the Ovid platform)
Embase (hosted on the Ovid platform)
Scopus
Cochrane
PubMed
CINAHL
All search results were exported and stored in EndNote 20 (Version 20.2.1, Clarivate, 2021) and duplicates were removed.
3: DATA EXTRACTION
Articles that passed both screening steps and met the eligibility criteria were then subjected to data extraction. Data (including study type, definitions of healing and non-healing, wound details, sample type, sample size, and levels and quality of evidence) was extracted in accordance with the data extraction tool (APPENDIX A). Despite the debate surrounding the use of quality assessment in scoping reviews,41,60 it was decided to systematically demonstrate that the quality of evidence collated was acceptable to enhance the validation of the results of this review. The QualSyst tool (APPENDIX B) proposed by the Alberta Heritage Foundation61 was decided upon given that it outputs a number providing a quantitative and reproducible means of identifying quality that other critical appraisal tools do not.62 The outputted score allows a source to be categorized as limited, adequate, good, or strong quality. Similarly, evidence levels were assessed using the JBI levels of evidence (APPENDIX C).
High numbers of poor-quality and low-level evidence could be considered indicative of a need for improvements in biomarker research methods.
4: DATA ANALYSIS AND PRESENTATION
The nature of a scoping review does not lend itself to a meta-analysis, thus it is recommended that it should instead focus on basic descriptive analysis such as frequency counts of concepts. Peter et al.35 further state that in some cases basic coding in a review proves useful particularly when identifying or clarifying definitions. Since the objective of this review requires the synthesis of wound healing definitions, coding is justified. To explore relationships between study types and definitions of healing and non-healings, results are subdivided into study types with frequency counts of definitions within these study types identified.
Extracted data is expressed in two primary formats. The first is a summary of the search results and selection process,35 including a PRISMA diagram. The second is the presentation of the data extracted from the included sources, in such a format that the research question is answered. Results are descriptively presented in paragraphs that align with the review's objectives and are diagrammatically mapped. Charts allow frequency counts to be graphically visualized. It is well-known that data visualizations enhance understanding.63 All charted data (including source references) are openly available in the review's dataset64 stored on the University of Strathclyde KnowledgeBase.
RESULTS
1: OVERALL RESULTS
1.1: Search Strategy Results and Included Articles
Of the 7,041 sources generated from the search strategy (Table 5), 3,735 were duplicates, so 3,306 titles and abstracts were screened (Figure 1). 2,659 sources were excluded, leaving 647 for full-text screening. After exclusion, 219 articles remained and were subjected to data extraction. Primary reasons for exclusion included review articles, unclear methodologies, no ethical approval, inaccessible texts, language constraints, irrelevant wound healing, and a lack of focus or discussion on biomarkers.
Table 5:
Breakdown of the search strategy results for each searched database.
| Database | Search Date | Number of Results | Limited to Abstracts, Titles, Keywords (specifics of the applied limit) | Limited to 2017 and after | |
|---|---|---|---|---|---|
| Web of Science | 06/05/2023 | 4,924 | 2,087 (abstract limit) | 931 | |
| Ovid Medline | 06/05/2023 | 2,942 | 2,852 (abstract limit) | 1,086 | |
| Ovid Embase | 06/05/2023 | 4,050 | 3,934 (abstract limit) | 1,818 | |
| Scopus | 06/05/2023 | 4,534 | 4,534 (title, abstract, keyword limit) | 1,828 | |
| PubMed | 06/05/2023 | 3,833 | 2,199 (title, abstract limit) | 916 | |
| CINAHL | 06/05/2023 | 1,014 | 505 (abstract limit) | 245 | |
| Cochrane | Cochrane Reviews | 06/05/2023 | 202 | 16 (title, abstract, keyword limit) | 8 |
| Cochrane Protocols | 06/05/2023 | 30 | 0 (title, abstract, keyword limit) | 0 | |
| Cochrane Trials | 06/05/2023 | 318 | 312 (title, abstract, keyword limit) | 209 | |
| Total References | 7,041 | ||||
| Duplicates Removed | 3,735 | ||||
| Total References to Screen | 3,306 | ||||
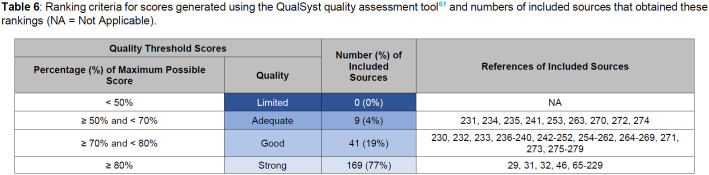
1.2: Quality and Levels of Evidence
All included evidence was quantitative with 77% of all studies29,31,32,46,65–229 demonstrating strong quality, and 0 studies graded with limited quality (Table 6). Evidence levels, on the other hand, varied more; for Prognosis 35 studies were graded level 1.b (the second highest level of evidence), and 4 (Table 7) were graded 5.c (the lowest level of evidence), whereas for Effectiveness, 1 and 12 studies were graded 1.b and 1.c, respectively. However, 98 studies were graded 5.c (Table 7).
Table 6:
Ranking criteria for scores generated using the QualSyst quality assessment tool61 and numbers of included sources that obtained these rankings (NA = Not Applicable).
| Quality Threshold Scores | Number (%) of Included Sources | References of Included Sources | |
|---|---|---|---|
| Percentage (%) of Maximum Possible Score | Quality | ||
| < 50% | Limited | 0 (0%) | NA |
| ≥ 50% and < 70% | Adequate | 9 (4%) | 231, 234, 235, 241, 253, 263, 270, 272, 274 |
| ≥ 70% and < 80% | Good | 41 (19%) | 230, 232, 233, 236–240, 242–252, 254–262, 264–269, 271, 273, 275–279 |
| ≥ 80% | Strong | 169 (77%) | 29, 31, 32, 46, 65–229 |
Table 7:
Levels of evidence of the included articles in accordance with the JBI Levels of Evidence (APPENDIX C) (JBI = Joanna Briggs Institute; NA = Not Applicable).
| Evidence Level | JBI Evidence Level Study Categories | |||
|---|---|---|---|---|
| Effectiveness | Diagnosis | Prognosis | ||
| 1.a | 0 | 0 | 0 | |
| 1.b | 1 (237) | 10 (75, 85, 94, 130, 144, 158, 176, 231, 232, 234) | 35 (29, 31, 68, 74–76, 81, 83, 85, 87, 94, 97, 100, 102, 124, 132, 144, 158, 171, 175, 176, 179, 191, 195, 198, 201, 203, 206, 230–236) | |
| 1.c | 12 (103, 105, 136, 163, 212, 238–244) | NA | NA | |
| 1.d | 0 | NA | NA | |
| 2.a | 0 | 0 | 0 | |
| 2.b | 0 | 0 | 0 | |
| 2.c | 0 | NA | NA | |
| 2.d | 0 | NA | NA | |
| 3.a | 0 | 0 | 0 | |
| 3.b | 1 (159) | 0 | 50 (32, 46, 72, 73, 84, 88, 90, 91, 98, 104, 106, 109–113, 118, 123, 125, 126, 128, 130, 138, 139, 141–143, 148, 149, 154, 156, 161, 166, 184, 185, 187, 188, 194, 197, 211, 213, 214, 216, 220, 274–279) | |
| 3.c | 3 (77, 232, 268) | NA | NA | |
| 3.d | 3 (151, 183, 269) | NA | NA | |
| 3.e | 34 (31, 69, 75, 76, 84, 99, 102, 106, 109, 113, 114, 116, 121, 122, 125, 130, 144, 149, 153, 158, 176, 179, 185, 196, 198, 199, 201, 224, 234, 235, 270–273) | NA | NA | |
| 4.a | 0 | 0 | 0 | |
| 4.b | 0 | 0 | 2 (93, 151) | |
| 4.c | 0 | NA | NA | |
| 4.d | 1 (89) | NA | NA | |
| 5.a | 0 | 0 | 0 | |
| 5.b | 0 | 0 | 0 | |
| 5.c | 98 (65–67, 70, 71, 78–80, 82, 86, 92, 95, 96, 107, 108, 115, 117, 119, 120, 127, 129, 131, 133–135, 137, 140, 145–147, 150, 152, 155, 157, 160, 162, 164, 165, 167–170, 172–174, 177, 178, 180–182, 186, 189, 190, 192, 193, 200, 202, 204, 205, 207–210, 215, 217–219, 221–223, 225–229, 245–267) | 4 (75, 85, 94, 231) | 4 (101, 127, 131, 178) | |
Study types additionally meant that no studies were graded for Meaning or Economic Evaluation levels of evidence. For levels of evidence, it is important to note that the total frequency counts add up to greater than 219 (the number of included articles) given that several studies were graded in more than one evidence level category; for example, often when graded for Prognosis, they were additionally graded for Effectiveness. Interestingly, 153 (70% of 219) were graded for Effectiveness, yet only 14 (6% of 219) met the criteria to be graded for Diagnosis (Table 7).
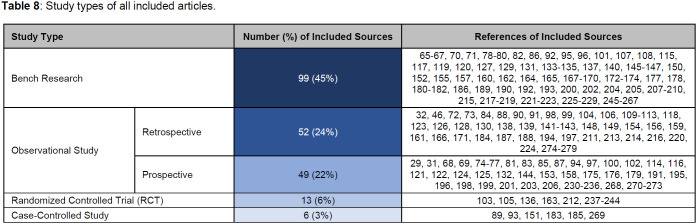
1.3: Study Types and Settings
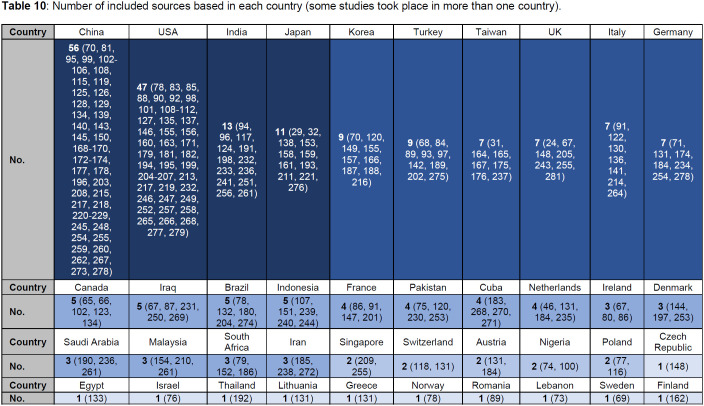
The most common study type was bench research, with 99 studies of this kind and only 6 case-controlled studies (Table 8). The most common setting research took place in was a university environment (190 studies), whereas only 1 study occurred in a governmental organization setting (Table 9). 66 and 35 studies were conducted in medical centers and research centers, respectively (Table 9). Note that the counts of settings and countries exceed 219 because 76 (35%) of the articles took place in more than one setting, and 26 (12%) of articles took place in more than one country. All included articles came from 40 countries, with 56 studies affiliated with China alone (Table 10). Whereas, only 7 and 3 articles were based in the UK and Ireland, respectively.
Table 8:
Study types of all included articles.
| Study Type | Number (%) of Included Sources | References of Included Sources | |
|---|---|---|---|
| Bench Research | 99 (45%) | 65–67, 70, 71, 78–80, 82, 86, 92, 95, 96, 101, 107, 108, 115, 117, 119, 120, 127, 129, 131, 133–135, 137, 140, 145–147, 150, 152, 155, 157, 160, 162, 164, 165, 167–170, 172–174, 177, 178, 180–182, 186, 189, 190, 192, 193, 200, 202, 204, 205, 207–210, 215, 217–219, 221–223, 225–229, 245–267 | |
| Observational Study | Retrospective | 52 (24%) | 32, 46, 72, 73, 84, 88, 90, 91, 98, 99, 104, 106, 109–113, 118, 123, 126, 128, 130, 138, 139, 141–143, 148, 149, 154, 156, 159, 161, 166, 171, 184, 187, 188, 194, 197, 211, 213, 214, 216, 220, 224, 274–279 |
| Prospective | 49 (22%) | 29, 31, 68, 69, 74-77, 81, 83, 85, 87, 94, 97, 100, 102, 114, 116, 121, 122, 124, 125, 132, 144, 153, 158, 175, 176, 179, 191, 195, 196, 198, 199, 201, 203, 206, 230-236, 268, 270–273 | |
| Randomized Controlled Trial (RCT) | 13 (6%) | 103, 105, 136, 163, 212, 237–244 | |
| Case-Controlled Study | 6 (3%) | 89, 93, 151, 183, 185, 269 | |
Table 9:
Setting in which the included articles took place. Note that university includes university hospitals and some sources took place in more than one setting.
| Setting | University | Medical Center | Research Center | Governmental Organization |
|---|---|---|---|---|
| Number of Included Sources | 190 | 66 | 35 | 1 |
| References | 29, 31, 32, 65–73, 75–81, 83–87, 89–93, 95–108, 110–113, 115–123, 125–157, 159, 161, 163–177, 179–182, 184–195, 197, 199–210, 212–229, 231–240, 243, 244, 246–250, 252–255, 259–269, 272, 273, 275, 277, 278 | 22, 29, 31, 32, 46, 72, 74, 76, 78, 82, 85, 88, 90, 91, 93, 99, 100, 105, 109, 114, 123, 125, 128–130, 136, 138, 139, 148, 151, 154, 158, 167, 172, 175, 179, 194–198, 201–203, 205–207, 211, 214, 216, 228, 230, 232, 239–242, 248, 253, 255, 257, 268, 273, 274, 276, 277, 279 | 94, 96, 124, 141, 150, 155, 157, 160, 162, 169, 173, 174, 178, 180, 183, 184, 198, 200–202, 206, 207, 215, 228, 241, 245, 251, 255, 256, 258, 264, 267, 268, 270, 271 | 114 |
Table 10:
Number of included sources based in each country (some studies took place in more than one country).
| Country | China | USA | India | Japan | Korea | Turkey | Taiwan | UK | Italy | Germany |
|---|---|---|---|---|---|---|---|---|---|---|
| No. | 56 (70, 81, 95, 99, 102–106, 108, 115, 119, 125, 126, 128, 129, 134, 139, 140, 143, 145, 150, 168–170, 172–174, 177, 178, 196, 203, 208, 215, 217, 218, 220–229, 245, 248, 254, 255, 259, 260, 262, 267, 273, 278) | 47 (78, 83, 85, 88, 90, 92, 98, 101, 108–112, 127, 135, 137, 146, 155, 156, 160, 163, 171, 179, 181, 182, 194, 195, 199, 204–207, 213, 217, 219, 232, 246, 247, 249, 252, 257, 258, 265, 266, 268, 277, 279) | 13 (94, 96, 117, 124, 191, 198, 232, 233, 236, 241, 251, 256, 261) | 11 (29, 32, 138, 153, 158, 159, 161, 193, 211, 221, 276) | 9 (70, 120, 149, 155, 157, 166, 187, 188, 216) | 9 (68, 84, 89, 93, 97, 142, 189, 202, 275) | 7 (31, 164, 165, 167, 175, 176, 237) | 7 (24, 67, 148, 205, 243, 255, 281) | 7 (91, 122, 130, 136, 141, 214, 264) | 7 (71, 131, 174, 184, 234, 254, 278) |
| Country | Canada | Iraq | Brazil | Indonesia | France | Pakistan | Cuba | Netherlands | Ireland | Denmark |
| No. | 5 (65, 66,, 102, 123,, 134) | 5 (67, 87, 231, 250, 269) | 5 (78, 132, 180, 204, 274) | 5 (107, 151, 239, 240, 244) | 4 (86, 91, 147, 201) | 4 (75, 120, 230, 253) | 4 (183, 268, 270, 271) | 4 (46, 131, 184, 235) | 3 (67, 80, 86) | 3 (144, 197, 253) |
| Country | Saudi Arabia | Malaysia | South Africa | Iran | Singapore | Switzerland | Austria | Nigeria | Poland | Czech Republic |
| No. | 3 (190, 236, 261) | 3 (154, 210, 261) | 3 (79, 152, 186) | 3 (185, 238, 272) | 2 (209, 255) | 2 (118, 131) | 2 (131, 184) | 2 (74, 100) | 2 (77, 116) | 1 (148), |
| Country | Egypt | Israel | Thailand | Lithuania | Greece | Norway | Romania | Lebanon | Sweden | Finland |
| No. | 1 (133) | 1 (76) | 1 (192) | 1 (131) | 1 (131) | 1 (78) | 1 (89) | 1 (73) | 1 (69) | 1 (162) |
2: DEFINITIONS
2.1: Healing Definitions
As depicted in Figure 2, 43% (n = 95) of included sources (all study types) provided no definition of healing. When definitions were provided, healing was most explained by complete epithelization/healing and change in wound area/size, utilised in 14% (n = 30) and 28% (n = 61) of sources respectively. Changes in wound area were most often used in bench research studies (92% of included sources using this definition) and were commonly presented as a wound healing rate defined as follows (Equation (1)):
Figure 2:
Frequency counts of healing definitions provided in all included sources, categorized by study types (OHP = hydroxyproline; DUSS = diabetic ulcer severity score; SINBAD = site, ischemia, neuropathy, bacterial infection, and depth; UT = University of Texas; DEPA = depth of ulcer, extent of bacterial colonization, phase of ulcer, and association etiology; PEDIS = perfusion, extent, depth, infection, and sensation; NA = not applicable). Note that the total frequency equates to greater than 219 (the number of total included sources) given that some included sources encapsulated two definitions in order to define healing (e.g. change in wound area and absence of wound complications).
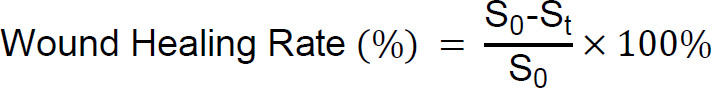 |
Where S0 is the original wound area, and St refers to the wound area at any given time after injury. Interestingly only one source242 incorporated biomarkers in their definition of healing, using OHP (hydroxyproline) levels as a surrogate marker of healing in their randomized control trial investigating the effects of topical negative pressure (TNP) therapy on tissue oxygenation and wound healing in vascular foot wounds.
A further 6 sources68,76,149,163,237,244 (all human participant studies) added a more systematic approach to the definition of healing than others through the implementation of grading systems/scales such as the Wagner Scale or the University of Texas classification system. Chen et al.,237 in their randomized controlled trial, defined a healed ulcer as Wagner Grade 0 (skin intact, but bony deformities lead to “foot at risk”282) and 1 (superficial ulcer). Lee et al.163 evaluated residual limb incision healing using a modified Bates-Jensen Score (mBJS) assessment tool, scoring the following criteria from 1 to 5: amputation skin color, epithelization, amount of exudate, and the presence and volume of eschar. Higher scores therefore indicate worse healing. In fact, Jeon et al.,149 in their observational retrospective study, employed and compared five classification systems for diabetic foot ulcers (Meggitt-Wagner classification; SINBAD [site, ischemia, neuropathy, bacterial infection, and depth] score; DEPA [depth of ulcer, extent of bacterial colonization, phase of ulcer, and association etiology] scoring system; UT [University of Texas] diabetic wound classification; DUSS [diabetic ulcer severity score]) to identify the “gold standard” prognostic classification system or optimum prediction tool for amputation.
2.2: Non-Healing Definitions
Over 80% (n = 183) of included sources provided no definition of impaired or non-healing wounds (Figure 3). In the limited sources (all were human participant studies) where a definition was stated, the identification of wound healing complications (Table 11), increase or no change in wound size, or the need for operative interventions, explained non-healing in 4% (n = 9),83,109,112,163,179,195,206,216,276 1% (n = 2),83,158 and 4% (n = 9)32,109,118,179,194,195,206,216, 273 of sources respectively. In none of the definitions were biomarkers used. Wound complications were defined differently depending on the source, as compiled in Table 11. Of the 9 sources using wound complications to define non-healing, 67% (n = 6) explored healing in relation to the amputation surgical site.109,112,163,206,216,267
Figure 3:
Frequency counts of non-healing definitions provided in all included sources, categorized by study types (NA = not applicable). Note that the total frequency equates to greater than 219 (the number of total included sources) given that some sources encapsulated two definitions (for example MacDonald et al.83 used no change in wound size and presence of wound complications) in order to define non-healing.
Table 11:
Wound healing complications stated in non-healing definitions coded for ‘wound complications’ (RCT = randomized controlled trial; CLTI = critical limb threatening ischemia; SSI = surgical site infection).
| Source | Study Type | Wound Type | Non-Healing Definition Wound Complications |
|---|---|---|---|
| Lee et al. (163) | RCT | Amputation | Signs such as erythema, drainage, infection, incision breakdown, skin/fat necrosis, and/or tissue eschar. |
| Majumdar et al. (179) | Observational Prospective | Surgical Site After Lower Extremity Revascularization | Need for operative interventions for SSI or dehiscence, or new ulcerative wound or bypass graft infection. |
| Nystrom et al. (195) | Observational Prospective | Surgical Site After Lower Extremity Soft Tissue Sarcoma Excision | Any wound-related issue (necrosis, dehiscence, infection, seroma) treated by a return to the operating room, initiation of oral or intravenous (IV) antibiotics, intervention for seroma including aspiration, or prolonged wound packing or dressing changes greater than 120 days. |
| Squiers et al. (206) | Observational Prospective | Lower Limb Amputation | Development of necrosis; development of infection, including gangrene or abscess; ulceration occurring within or adjacent to the surgical wound; disruption or dehiscence of suture line; drainage or exudate expressed from the suture line; evidence of inflammatory response including swelling, cellulitis, or skin discoloration; hematoma formation; revision of the amputation to a more proximal level. |
| MacDonald et al. (83) | Observational Prospective | Diabetic Foot Ulcer | Pain, erythema, oedema, heat, purulent exudate, serous exudate with concurrent inflammation, delayed healing, discoloration of granulation tissue, friable granulation tissue, pocketing at the base of the wound, foul odor, and wound breakdown. |
| Adams et al. (109) | Observational Retrospective | Transmetatarsal Amputation | (1) revision of the amputation, defined as a return to the operating room for any reason; (2) postoperative infection, defined as any superficial or deep infection requiring oral antibiotics, admission to the hospital for intravenous antibiotics, and/or an unplanned return to the operating room; (3) chronic residual limb ulceration, defined as a non-healing wound at the surgical site requiring >4 weeks of wound care; (4) calcaneal gait, defined as any increased pressure at the plantar heel resulting in a pressure sore; (5) residual limb deformity, defined as a nonplantigrade foot; and (6) residual limb infarction, defined as ischemia or necrosis of the incision site. |
| Alfawaz et al. (112) | Observational Retrospective | Below-Knee Amputation | Separation or necrosis of skin, flap necrosis, or dry ischemic eschar formation. |
| Morisaki et al. (276) | Observational Retrospective | Above or Below-Knee Amputation | Surgical site infection or wound dehiscence. |
| Woo et al. (216) | Observational Retrospective | CLTI Patient Ulcer or Amputation Surgical Site | Wounds requiring regular dressing and antibiotic treatment or surgical wound revision and additional surgery. |
In addition to major amputation, Furuyama et al.32 further defined ulcer non-healing in patients with critical limb ischemia by amputation or death. Contrastingly Kimura et al.158 defined worsened foot wounds only as wounds that had increased in size without amputation, with participants resulting in minor or major amputation, or all-cause death in the one-year study period being classified separately.
In 10% (n = 22) of all included sources80,85,86,88,94–96,106,121,127,144,145,169,178,183–185,205,207,234,253,279 both healing and non-healing definitions were considered not applicable given the content of the research. Laiva et al.,80 for example, explore the expression of pro-angiogenic factors (characteristic of wound healing) in human diabetic adipose-derived stem cells cultured on collagen scaffolds. Although this is investigating aspects of ulcer healing and is therefore relevant to the scoping review research question, it focuses on a specific cellular aspect of non-healing diabetic foot ulcers (DFUs), rather than in vivo whole ulcer healing (where several tissues and cells are involved).
DISCUSSION
This scoping review aimed to compile definitions of healing and non-healing found in the literature investigating biomarkers of healing in the tissues and structures of residual limbs of adults with amputation. The findings indicate a significant lack of standardized definitions of healing within the literature, with only one source242 incorporating biomarkers (an objective measure rather than a subjective one) to define healing. Systematic methods for quantifying healing, such as pre-defined grading systems or scales like the Wagner Scale, were utilized in only 2% of the studies included. Moreover, these tools are generally designed for the assessment of open wound healing rather than surgical site healing. Similarly, definitions of non-healing were either absent or inconsistently characterized by varying descriptions of wound complications.
The review highlights a broader lack of consensus and standardization in defining both healing and non-healing, as current definitions are often superficial and predominantly based on visual and size-based assessments. These approaches fail to consider deeper tissue healing and mechanical properties essential for functionality, particularly in the context of prosthesis use. There is a critical need for more comprehensive, multidimensional definitions that incorporate objective measures like biomarkers and mechanical assessments, along with social and psychological evaluations, to more accurately reflect the complex nature of healing to guide future research and clinical practice more effectively.
1: OVERALL SEARCH RESULTS
No set number of articles should or should not be included in a review,283 and the number of included articles comes down to the search strategy and inclusion criteria. In this review, an arguably large number of articles (219) met the inclusion criteria, whereas in the similar work by Day et al.16 on determinants of healing and readiness for prosthetic fitting after transtibial amputation, 2,067 articles met the search strategy yet only 20 passed both screening stages. This difference is likely due to their inclusion criteria of transtibial amputation; in this review with the knowledge that the literature on healing on amputation specifically is low, the research question was expanded to wound healing of tissues like that of the lower limb residuum, thus broadening the number of search results.
Of the 195 countries in the world, research from 40 of these countries was included in this review, several of which were LMICs (low-to-middle-income countries such as Cuba, Egypt, China, Malaysia, Nigeria, Thailand, and Pakistan).284 Such global research allows us to expand findings across populations, regions, and cultures,285 reduces Western publication biases, and is critical in overcoming global health challenges286 like wound healing. It can be argued that the high number of countries from which research in this review originates highlights the global burden of wound healing. This is reinforced by the reported average of $2.8 billion spent globally on wound healing in 2014.287 Guest et al.288 concluded that in the UK alone, between 2017 and 2018, the cost to the NHS per healed wound ranged from £698 to £3,998 per patient, and that of an unhealed wound ranged from £1,719 to £5,976 per patient.
Interestingly, Tricco et al.,289 in their scoping review of scoping review methodologies, revealed that 423 (86%) of the articles that met their inclusion criteria did not use a quality appraisal tool in their scoping review. However, it is well reported that critical (or quality) appraisal tools are a justifiable addition to a review to systematically assess the credibility of the research on which the results of the scoping review are then based.290 On the other hand, Tod et al.290 further note that quality checklists, like the QualSyst tool, lack evidence to support their use; thus, quality assessment acts as an outcome measure, not an exclusionary criterion in this review.
As detailed in the results section (Quality and Levels of Evidence) the high number of Effectiveness 5.c levels of evidence can be attributed to the 99 bench research studies (almost 50% of the included articles) that were included in data extraction. Of the 99 studies, 81 were rat or mouse studies, reinforcing the justification of bench research receiving the lowest level of evidence following the JBI levels of evidence. The lower number of higher-level evidence studies can be explained by the cost of studies such as RCTs (estimated to cost anywhere in the range of $43 to 103,254 per patient),291 and the common lag (as long as 17 years) in translating scientific discoveries (produced through bench research) into patient studies and thus patient benefit.292
2: DEFINITIONS OF HEALING AND NON-HEALING
In their review of complete wound closure definitions, Gould and Li28 recorded that complete/full/100% (re)epithe-lialization or closure was the most common definition of healing. The same was noted here, of the 102 sources (47% of all included sources) that provided definitions of healing, 30 were regarding epithelialization, and 61 were defined by changes in wound size/area. However, this assessment is limited in its applicability, particularly for surgical sites, such as amputation, which do not involve open wounds. The reliance on wound size to indicate healing, particularly through methods like measuring with disposable tapes,293 is problematic due to poor inter-rater and intra-rater reliability, its time-consuming nature, and issues inaccuracy.294–296 Importantly, this focus on epithelialization alone does not capture the entirety of the healing process, as the proliferation phase, in which epithelialization occurs, is only one of four phases of wound healing. Re-epithelialization occurs in the third phase, the proliferation phase (which takes place days to weeks after injury), where granulation tissue is formed, the epidermis is regenerated and neovascularization occurs.2 This phase is then followed by the fourth and final phase which occurs weeks to months after injury, remodeling, characterized by vascular maturation and regression, collagen remodeling, and the point at which a wound reaches its maximum strength and ultimate endpoint.2,3 In cutaneous tissue for example this final phase is marked by a collagenous scar. Therefore, it can be argued epithelialization suggests healing but does not indicate a fully healed wound. Particularly in the case of an amputation where the suture line may appear healed after re-epithelization has occurred, but the final phase of healing is still taking place below the skin and is likely heavily influenced by prosthetic use (and its subsequent mechanical loading).24 For example, Bramley et al.8 conducted a study investigating changes in tissue composition and load response on 10 individuals with unilateral transtibial amputations, who had undergone the procedure between 1 and 35 years prior to the study (mean of 7.5 years) and were therefore classified as having mature residual limbs.25 The findings indicated a higher presence of adipose tissue infiltrating the muscle in residual limbs compared to intact contralateral limbs, suggesting muscle atrophy and adaptation post-amputation.8 Furthermore, intramuscular adipose content was found to correlate negatively with daily prosthetic socket use, reinforcing the idea that prosthetic use influences tissue composition in mature residual limbs, and likely has an even greater impact on early healing residual limbs. Therefore, a more comprehensive approach to defining healing should consider the deeper, ongoing processes beyond surface closure.
Definitions of non-healing were more infrequent and when provided were complex, typically focusing on the identification of complications or deviations from normal healing. One possible reason for the limited reporting of non-healing definitions is the assumption by researchers that by defining healing, non-healing is implicitly understood as the opposite. Or perhaps the challenge of clearly defining non-healing is a symptom of the complexity of a chronic wound, its causes, and the variety of systemic (for example age,297, sex hormones,298 alcoholism,299 smoking,300 and nutrition301) and local (for example infection,302 oxygenation,303 and venous sufficiency304) factors that impact healing.4 It is noteworthy that among the sources surveyed, studies focusing on amputation surgical sites were the primary providers of definitions for non-healing wound complications (6 of 9 included sources 83,109,112,163,179,195,206,216,276). This trend may arise from the fact that traditional definitions of open wound healing, like epithelization or wound site evaluation, do not readily apply to closed surgical site wound types. Furthermore, individuals undergoing amputation often present with multiple comorbidities, such as diabetes and peripheral vascular diseases,19 and systemic factors for non-healing, such as smoking and alcohol use,305,306 which can negatively impact the healing process.20,299,300,307 For instance, Lind et al.306 retrospectively examined the impact of smoking on post-operative complications in 137 patients who had undergone primary above-knee or below-knee amputations, 44 of whom were cigarette smokers. The study found that smokers had a 2.5 times higher risk of infection and re-amputation compared to non-smokers, concluding that abstaining from smoking during the post-operative healing phase is critical, as nicotine compromises cutaneous blood flow velocity and increases the risk of microthrombus formation.306 It can also be argued that healing complications such as infection or excessive oedema are primary barriers to prosthetic readiness, and thus of greater concern to prosthetists and rehabilitation professionals than indicators of healthy healing. Churilov et al.,308 for example, observed that the use of rigid dressings post-transtibial amputation, hypothesized to reduce swelling and promote healing, significantly shortened the time from amputation to casting or fitting of the first prosthesis, compared to traditional soft elastic dressings. In summary, identifying abnormal healing processes, particularly in the context of amputation, requires a more comprehensive approach than surface level visual assessments. A standardized system, tailored to specific wound types, would improve the clarity and consistency of healing and non-healing definitions.
A biomarker, however, would allow both healing and non-healing to be defined and monitored objectively and quantitatively. Unfortunately, only one included source242 considered a biomarker in their definition of wound healing stating that they were to “demonstrate the effects of TNP on the healing of acute wounds of the foot by measuring the change in wound volume and collagen deposition”, enlisting OHP as a well-reported surrogate marker of collagen.242 In addition to deposition during the proliferative phase of healing, collagen, a key component of the extracellular matrix, induces platelet activation and aggregation in response to injury (phase one of healing), promotes fibroblast recruitment in the inflammatory stage, and influences remodeling of the extracellular matrix (ECM) increasing the tensile strength of the wound in the final remodeling/maturation phase.309 Chiang et al.242 further reported that wound volume reduction from day 0 to day 14 of treatment was not significant (44.2% TNP vs 20.9% control; p = 0.15) suggesting that TNP did not expedite wound healing as expected. Similarly, the degree of collagen deposition (OHP content in tissue samples was expressed in micrograms of collagen per milligram of granulation tissue) on day 14 was also not significant between control and TNP-treated groups (58% TNP vs 94.5% control; p = 0.32).242 In terms of absolute values, the TNP group noted a larger reduction in wound size, but the control group observed a greater increase in collagen deposition. This reinforces the notion that there is more to the healing process than simply the dimensions of the open wound. Thus, biomarkers could provide a more nuanced and objective means of tracking both healing and non-healing across all wound types, including surgical sites. Biomarkers have also been demonstrated in osteoarthritis research to indicate responses to loading tasks, providing valuable insights into joint health and predicting structural changes.310 This knowledge could be applied to monitoring the health of the residual limb, which undergoes adaptation during healing and early prosthetic use. For instance, in a posterior flap below-knee amputation, the gastrocnemius muscle forms a significant part of the muscle bulk covering the residual tibia. During prosthetic use, this muscle is subjected to forces in directions it would not experience in an intact limb, necessitating adaptation in response to these forces.
Although not utilizing biomarkers, definitions in 6 sources68,76,149,163,237,244 appeared to adopt a more systematic approach to assessing healing through the use of scales and classification systems such as the Wagner (or Meggitt-Wagner) system. Bar the modified Bates-Jensen (mBJS) adopted by Lee et al.,163 the classifications used apply only to diabetic open wounds or ulcers and again rely only on visual/surface level assessment external to the wound, limiting their relevance to surgical wounds. Diabetic foot ulcers (DFUs) account for much of the research on wound healing due to their global burden, with 80% of lower extremity amputations (LEAs) linked to DFUs.311 However, overemphasizing DFUs risks overlooking the specific needs of amputation sites, which require different criteria for assessing healing. The aforementioned mBJS which evaluates necrotic tissue topes, necrotic tissue volume, exudate type, skin color surrounding the wound, and epithelialization on a scale of 1 (best healing) to 5 (worst healing),312 although designed specifically for residuum healing assessment, is also limited to observer interpretation of the surgical site. Though not used in included sources, further surgical site healing classifications exist like the Centers for Disease Control (CDC) Surgical Wound Classification (SWC)313 and the Surgical Wound Assessment Tool (SWAT),314 but again they incorporate only a variety of subjective observations and are focused primarily on the identification of surgical site infections only. Despite being more holistic tools, these classifications still provide only subjective indicators of what is occurring under the skin and are therefore limited in truly assessing deep tissue healing; limitations that could be solved with more objective measures like biomarkers.
Interestingly, all the definitions of healing and non-healing focus purely on the physical components of wound healing. The optimal healing environments (OHE) framework however suggests that patient healing is best supported by addressing not just the physical, but the social, psychological, spiritual, and behavioral components of healthcare.315 Doering et al.,316 for example, observed that in 72 patients with bypass surgery, those with higher depressive symptom scores (indicating more symptoms) reported poorer emotional recovery (p < 0.001) and poorer physical recovery (p = 0.007) and achieved shorter walking distances (p <0.001) than did patients with lower scores (indicating fewer symptoms). Furthermore, by 6 weeks after discharge, infections and impaired wound healing were more common among patients with higher depressive symptom scores (46%) than among patients with lower scores (19%, p = 0.03).316 Similarly, it is well known that amputation has psychological effects, with one review revealing that across 12 studies the prevalence of psychiatric disorders among amputees in India is in the range of 32% to 84%, including depression rates of 10.4% to 63% of the studied population, posttraumatic stress disorder rates of 3.3% to 56.3%, and phantom limb phenomenon rates of 14% to 92%.317 These symptoms of anxiety and depression reportedly do improve over time,317,318 yet no definitions of amputation healing detailed in this scoping review alluded to anything other than the physicality of the surgical site. Perhaps in the future, more effort should be made to consider more than the physical aspects when defining healing, providing a more holistic definition of healing.315,319,320 An amputation is a life-changing event; with more objective and well-explained definitions of healing individuals with amputations may feel more comfortable about their surgical site healing journey which is currently limited by biases introduced by the timing of clinician visits and subjective surface level wound examination only.16,321
Overall, the lack of provided definitions, irrespective of evidence level, wound type, or study type, raises concerns. For example, 13 included sources were RCTs (Table 8), the highest level of evidence, yet of these only 6 and 1 provided healing163163,237,238,241,242,244 and non-healing163 definitions respectively. Despite investigating healing, or an aspect of it, by not defining healing and non-healing the methodological rigor of the study is reduced by not providing a clear endpoint definition, and the belief that assessing wound healing is a purely visual process is perpetuated. As noted in previous studies16,321 the gap in the literature on healing definitions, particularly for amputation sites, remains unaddressed for over 20 years, despite its significance to patient outcomes. A shift toward more objective, comprehensive measures, incorporating biomarkers, psychological factors, and standardized definitions, would greatly enhance the study of wound healing in clinical settings.
To develop a tailored and relevant scale for assessing wound healing in the context of residual limbs post-amputation, the authors believe the following considerations should be made to ensure that it is comprehensive, objective, and clinically useful:
Incorporate all four phases of healing, capturing both surface level and deeper tissue healing processes.
-
Incorporate objective measures like biomarkers:
This will require identifying the most appropriate biomarkers for assessing post-amputation healing, potentially through a scoping review or bench research. For example, determining which biomarkers best assess the residual limb's capacity to withstand prosthetic fitting could include indicators of healing complications like infection, inflammation, cell death, or response to mechanical loading. Song et al.322 identified that inflammatory markers such as white blood cell count, serum C-reactive protein levels, and erythrocyte sedimentation rate were significantly correlated with wound healing rates in diabetic patients. Additionally, thresholds or cut-off values for these biomarkers should be established to differentiate between healing and non-healing. For instance, a transcutaneous oxygen pressure (TcPO2) value below 40 mmHg has been associated with a 24% increased risk of healing complications in lower limb amputations, compared to values above 40 mmHg.323
Techniques to quantify these biomarkers must be developed or adapted. This could involve quantitative imaging techniques such as ultrasound, which has been used to observe deeper tissue changes and predict the prognosis of pressure injuries,324 or innovative tools like wearable smart bandages capable of sensing wound pH, temperature, bioimpedance, glucose, oxygen, proteins, or uric acid in real-time.325
-
Include subjective and psychosocial factors:
Psychological markers, such as anxiety, depression, and body image perception, should be addressed, as they influence overall recovery.316,317 A number of existing validated tools used in the lower limb amputee population are available such as the Hospital Anxiety and Depression Scale.326,327
Patient-reported outcomes (PROs), such as the Visual Analogue Scale (VAS) for pain328 and the Prosthetic Limb Users Survey of Mobility (PLUS-M),329 can capture the patient's perspective on pain, mobility, and comfort, offering deeper insights into functional recovery and prosthetic readiness. Research should explore which outcome measures most effectively reflect prosthetic readiness, perhaps through a pilot study investigating the effectiveness of different measurement tools in monitoring post-amputation healing. The COMET (Core Outcome Measures in Effectiveness Trials) initiative provides a list of key outcome measures for studies of people undergoing major lower limb amputation for complications of peripheral vascular disease, including death, quality of life, mobility, and social integration/independence,330 which can serve as a foundation to be built upon with more objective measures like biomarkers.
A multi-tiered grading system should be created, where each grade corresponds to specific milestones in the healing process, defined by clear criteria. For instance, Gethin et al.331 conducted a scoping review and identified normal wound bed temperature in chronic wounds as being between 30.2°C and 33.0°C. For each criterion, clear healing versus non-healing indicators should be established, distinguishing between successful healing and complications such as infection or excessive oedema. This will require participant research to identify objective indicators of both healthy (e.g., a decrease in temperature and pH332) and unhealthy (e.g., an increase in inflammatory markers333) healing processes. The classification system must undergo rigorous pilot testing and validation. This includes:
Reliability testing, ensuring high inter-rater and intra-rater reliability through testing in diverse clinical settings.
Construct validity testing, comparing the system against known standards to confirm its accuracy.
Patient-centered validation to ensure that users' opinions are incorporated during all stages of development to guarantee the scale addresses meaningful aspects of their recovery journey.334
In the future, automation and streamlined assessment processes could be explored, for example, potentially incorporating wearable sensors for remote monitoring of residual limb health during healing. This could enhance the scale's practicality and accessibility. It is also essential that the scale should undergo longitudinal tracking, allowing for continuous feedback and refinement. Regular updates or revisions should be made based on new research or clinical findings to reflect the evolving understanding of wound healing. By incorporating these elements, the scale will be robust, adaptable, and capable of providing both clinicians and patients with valuable insights into the healing process and readiness for prosthetic use.
3: METHODOLOGICAL DISCUSSION
3.1: Methodological Strengths
A scoping review appears to be the most suitable approach to answering the research question due to its ability to comprehensively explore the extensive and unclear literature on impaired and healthy wound healing biomarkers and definitions, without restrictions on source types. In contrast, a systematic review would necessitate a more narrowly defined research question.
A key strength of this review is simply the significance of the conclusions drawn. By highlighting both the lack of healing definitions and the limitations within provided definitions, this systematically implemented review reinforces the need for further research into objective measures to quantify healing. The sooner we can reach a consensus on the most appropriate definition of healing (both cutaneous and subcutaneous), the sooner we can identify or predict a healing/non-healing wound, and the sooner it can be prevented or treated.22
3.2: Methodological Limitations
Despite the implementation of an exhaustive search strategy, there is always a likelihood that some sources may have been missed. Therefore, it is important to remember that the results of the scoping review will guide future work; they will not influence healthcare policy, for example.
A further limitation is the current lack of a second reviewer contradicting the JBI's recommendation for a minimum of two reviewers to validate results, remove bias,35 and increase the number of relevant articles included in a review.335 However, given the nature of the authors' resource constraints, only the primary author of this study could act as the reviewer, and the supervisory team acted as a verifier. Again, it is important to consider the purpose of the review;336 for example, is it impacting policy? If so, then it is particularly pertinent to ensure the methodology and the inclusion/exclusion criteria are rigorously justified and piloted. The review reported here, although thorough, is not intended to directly impact policy, and the lack of a second reviewer is perhaps more justified. Furthermore, this is not too dissimilar to peer-reviewed and published scoping reviews, with the work of Tricco et al.289 (a scoping review of scoping reviews) revealing that only 34% of reviewed scoping reviews included two or more independent reviewers. Yet simply introducing a standardized data extraction form, as the review reported here did, can minimize bias.35
In the future, it would be beneficial to consider using multi-lingual reviewers given that only sources in or translated into the English language could be investigated, potentially increasing Western publication biases.54,55 The choice was made to refrain from utilizing online translation software due to the potential risk of semantic loss. Van Nes et al.337 for example recommend the use of a professional translator given that translation is an interpretative act in which meaning can be lost. However, this option is costly and falls beyond the scope of the research supporting this manuscript. Although including all study types ensures more relevant sources are captured, the inclusion of rodent studies and mathematical models can be questioned.
In review studies, a balance between high precision (narrow) and high recall (broad) searches is necessary to ensure sufficient studies are captured by the search whilst the time required to screen all included articles is feasible.60 As such, this step was deemed unfeasible; assuming 300 articles were included with 100 references each, a further 30,000 articles would need to be screened; this was considered not an option given the limited project timescale of the primary author.
Please note that a registered and published protocol for this review is not available, which may influence the consistency and transparency of the review process.
4: ETHICAL CONSIDERATIONS
The use of grey literature in reviews is a contentious topic. Searching for it can be time-consuming and it lacks the validation peer-reviewed literature can provide; however, it can reduce publication bias given that it provides data that is not found in commercially published articles.338 Thus, this review did aim to include grey literature however all that was generated during the searches did not meet the inclusion criteria; often failing to provide a sufficiently clear methodology and clear ethical approval.
RCTs are considered the highest level of evidence,280 however, they are expensive, and funding is limited. They are often industry-funded and therefore more likely to report a statistically significant positive outcome than studies without industry funding.339 Thus, evidence level has not been used as an exclusionary criterion in this scoping review. It was a requirement, however, that all included articles, where applicable, clearly stated ethical approval and sought informed consent when human participants were involved.
CONCLUSION
The aim of this review was to compile definitions of healing and non-healing provided in the literature investigating biomarkers of healing of the tissues and structures found in the residual limbs of adults. Wound healing was predominantly characterized by epithelization and wound closure, including healing rates, or left undefined. Non-healing was often poorly explained, typically assessed by the need for operative intervention including re-amputation or signs of impaired healing when defined. This review highlights shortcomings in current definitions of healing and non-healing, which are frequently absent or based on superficial assessments influenced by clinician perspectives. These definitions mistakenly equate wound appearance and size with healing at deeper tissue levels, neglecting to account for the mechanical properties of the tissue that are critical, particularly in tissue subjected to loading during lower limb prosthesis use. This underscores the need for a more comprehensive approach to wound healing assessment, integrating biomarkers and potentially incorporating social and psychological evaluations, as a patient's environment significantly impacts their healing process. Before we can enhance wound management both before and after amputation and expedite the return to daily activities, it is essential to establish a clear consensus on what defines the healing and non-healing processes.
DECLARATION OF CONFLICTING INTERESTS
The author has no conflicts of interest to declare.
AUTHORS CONTRIBUTION
Hannelore Williams-Reid: the primary author of the manuscript, undertook the scoping review and prepared the final manuscript as part of a 4-year PhD program.
Arjan Buis: the primary PhD supervisor, assisted in developing the scoping review methodology and preparing the manuscript for publication.
Anton Johannesson: the secondary PhD supervisor, assisted in developing the scoping review methodology and preparing the manuscript for publication.
All authors have read and approved the final version of the manuscript.
SOURCES OF SUPPORT
The PhD project under which this scoping review/manuscript falls is funded by the UKRI EPSRC as part of the Centre of Doctoral Training (CDT) in Prosthetics and Orthotics (P&O) (studentship 2755854 “Wound management and early prosthetic rehabilitation” within project EP/S02249X/1) and by Össur.
ACKNOWLEDGEMENTS
The author of this article would like to express appreciation to the Strathclyde Body Device Interface Mechanobiology Research Group for their assistance in the discussion of the review's methodology.
Abbreviations & Acronyms:
Abbreviation & Acronym: Definition
- CDC
Centers for Disease Control
- CLTI
Critical Limb Threatening Ischemia
- COMET
Core Outcome Measures in Effectiveness Trials
- CRP
C-Reactive Protein
- DEPA
Depth of ulcer, Extent of bacterial colonization, Phase of ulcer, and Association etiology
- DFU
Diabetic Foot Ulcer
- DTI
Deep Tissue Injury
- DUSS
Diabetic Ulcer Severity Score
- ECM
Extracellular Matrix
- FDA
Food & Drug Administration
- HIC
High Income Country
- IV
Intravenous
- JBI
Joanna Briggs Institute
- LEAs
Lower Extremity Amputation
- LMICs
Low to Middle Income Countries
- mBJS
Modified Bates-Jensen Score
- MMPs
Matrix Metalloproteinases
- n
Number
- NA
Not Applicable
- NHS
National Health Service
- NICE
National Institute for Health and Care Excellence
- OHE
Optimal Healing Framework
- OHP
Hydroxyproline
- PEDIS
Perfusion, Extent, Depth, Infection, and Sensation
- PLUS-M
Prosthetic Limb Users Survey of Mobility
- PRISMA-ScR
Preferred Reporting Items for Systematic Review and Meta-Analyses for Scoping Reviews
- PROs
Patient Reported Outcomes
- RCTs
Randomized Controlled Trials
- SINBAD
Site, Ischemia, Neuropathy, Bacterial Infection, and Depth
- SSI
Surgical Site Infection
- SWAT
Surgical Wound Assessment Tool
- SWC
Surgical Wound Classification
- TcPO2
Transcutaneous Oxygen Pressure
- TNP
Topical Negative Pressure
- UK
United Kingdom
- USA or US
United States of America
- UT
University of Texas
- VAS
Visual Analogue Scale
Appendix
APPENDIX A:
Table A.1.
(adapted from References 48 and 53): Data extraction tool used to extract data from all sources that passed both screening steps. NAST refers to data extraction categories that may not be applicable to all source types.
| Data to be Extracted | Clarification of Data Extraction Category |
|---|---|
| Scoping Review Details | |
| Scoping Review Title | Wound Management, Healing, and Early Prosthetic Rehabilitation: Part 1 - A Scoping Review of Healing and Non-Healing Definitions |
| Review Objectives | Summarized in Manuscript Section 1 (Introduction) |
| Review Questions | |
| Evidence Source Details and Characteristics | |
| Citation Details | Full Harvard APA 7th edition citation for the source including source URL. |
| Study Type | For example, an observational retrospective or case-controlled study. |
| Country | The geographical location where the source was generated. |
| Setting | For example, a hospital/medical Center, university, or research center. |
| One Sentence Summary | Summary of the study in one sentence. |
| Details/Results Extracted from the Sources of Evidence | |
| Participant Characteristics | For example, age range, gender, and comorbidities. Includes control group characteristics also. |
| Sample Type and Size | Refers to the number and type of participants investigated in the source (NAST). |
| Wound Details | This includes any details about the wound type, such as classification, average size, and burn or ulcer. |
| Follow-up Time | Refers to the time between or after reported outcome measures (NAST). |
| Definition of Healing | Definitions and terms are given for healthy (non-impaired) healing. |
| Definition on Non-Healing | Definitions and terms are given for unhealthy (impaired) healing. |
| Chemical Biomarkers Discussed | All chemical biomarkers discussed/measured in the source must be recorded here. See Manuscript Introduction for a definition of chemical. |
| Physical Biomarkers Discussed | All physical biomarkers discussed/measured in the source must be recorded here. See Manuscript Introduction for a definition of physical. |
| Other Biomarkers Discussed | All remaining biomarkers that do not fall into the chemical or physical category must be recorded here. |
| Biomarker Measurement Techniques | Summary of the discussed/used/described biomarker measurement techniques used in the source. |
| Outcome Measures | Reported outcome measures (aside from aforementioned biomarkers); for example, 3-year mortality may be the primary outcome measure. |
| Significant Results | Results of importance as judged by the reviewers. |
| Limitations | Key limitations of the biomarkers, biomarker quantification technologies, or methodologies that are explicitly mentioned in the source. |
| Level of Evidence | Level of evidence according to the JBI classification (Reference 280) (see Appendix C). |
| Quality of Evidence | Quality of evidence score generated using the QualSyst tool (Reference 61) (Appendix B). |
APPENDIX B:
Table B.1.
(Reference 61): QualSyst tool checklist for assessing the quality of quantitative studies. Note that NA is not an option for Criteria 1, 2, 4, 13 and 14. Each response is assigned a point score depending on how well it meets the criteria (“yes” = 2 points, “partial” = 1 points, and “no” = 0 points). Items not applicable to a certain study design are labelled as NA and excluded from the total score. A summary score is calculated by summing the total score and dividing by the possible score (the possible score is the maximum score (28 points) minus the number of “NA” responses multiplied by 2).
| Criteria | Yes (2) | Partial (1) | No (0) | NA | |
|---|---|---|---|---|---|
| 1 | Question/objective sufficiently described? | ||||
| 2 | Study design evident and appropriate? | ||||
| 3 | Method of subject/comparison group selection or source of information/input variables described and appropriate? | ||||
| 4 | Subject (and comparison group, if applicable) characteristics sufficiently described? | ||||
| 5 | If interventional and random allocation was possible, was it described? | ||||
| 6 | If interventional and blinding of investigators was possible, was it reported? | ||||
| 7 | If interventional and blinding of subjects was possible, was it reported? | ||||
| 8 | Outcome and (if applicable) exposure measure(s) well defined and robust to measurement/misclassification bias? Means of assessment reported? | ||||
| 9 | Sample size appropriate? | ||||
| 10 | Analytic methods described/justified and appropriate? | ||||
| 11 | Some estimate of variance is reported for the main results? | ||||
| 12 | Controlled for confounding? | ||||
| 13 | Results reported in sufficient detail? | ||||
| 14 | Conclusions supported by the results? | ||||
Table B.2.
(Reference 340): Ranking criteria for scores generated using the QualSyst quality assessment tool. The tool consists of a quantitative study checklist with 14 criteria. Each criterion can be scored 0, 1, or 2 points provided the study doesn't, partially does, or does meet the criteria respectively. Thus, the greater the score the higher the quality of evidence.
| Study Type | Maximum Possible Score | Quality Threshold Scores | |
|---|---|---|---|
| Percentage (%) of Maximum Possible Score | Quality | ||
| Quantitative | 28 | < 50% | Limited |
| ≥ 50% and < 70% | Adequate | ||
| ≥ 70% and < 80% | Good | ||
| ≥ 80% | Strong | ||
APPENDIX C:
Table C.1.
(Reference 280): JBI levels of evidence for effectiveness. These levels are intended to be used alongside the supporting document outlining their use and using these levels does not preclude the need for careful reading, critical appraisal and clinical reasoning when applying evidence.
| Levels of Evidence – Effectiveness | |
|---|---|
| Level 1 – Experimental Designs | Level 1.a – Systematic review of Randomized Controlled Trials (RCTs) |
| Level 1.b – Systematic review of RCTs and other study designs | |
| Level 1.c – RCT | |
| Level 1.d – Pseudo-RCTs | |
| Level 2 – Quasi-Experimental Designs | Level 2.a – Systematic review of quasi-experimental studies |
| Level 2.b – Systematic review of quasi-experimental and other lower study designs | |
| Level 2.c – Quasi-experimental prospectively controlled study | |
| Level 2.d – Pre-test – post-test or historic/retrospective control group study | |
| Level 3 – Observational – Analytic Designs | Level 3.a – Systematic review of comparable cohort studies |
| Level 3.b – Systematic review of comparable cohort and other lower study designs | |
| Level 3.c – Cohort study with control group | |
| Level 3.d – Case-controlled study | |
| Level 3.e – Observational study without a control group | |
| Level 4 – Observational – Descriptive Studies | Level 4.a – Systematic review of descriptive studies |
| Level 4.b – Cross-sectional study | |
| Level 4.c – Case series | |
| Level 4.d – Case study | |
| Level 5 – Expert Opinion and Bench Research | Level 5.a – Systematic review of expert opinion |
| Level 5.b – Expert consensus | |
| Level 5.c – Bench research/single expert opinion | |
Table C.2.
| Levels of Evidence – Diagnosis | |
|---|---|
| Level 1 – Studies of Test Accuracy Among Consecutive patients | Level 1.a – Systematic review of studies of test accuracy among consecutive patients |
| Level 1.b – Study of test accuracy among consecutive patients | |
| Level 2 – Studies of Test Accuracy Among Non-Consecutive Patients | Level 2.a – Systematic review of studies of test accuracy among non-consecutive patients |
| Level 2.b – Study of test accuracy among non-consecutive patients | |
| Level 3 – Diagnostic Case Control Studies | Level 3.a – Systematic review of diagnostic case control studies |
| Level 3.b – Diagnostic case-control study | |
| Level 4 – Diagnostic Yield Studies | Level 4.a – Systematic review of diagnostic yield studies |
| Level 4.b – Individual diagnostic yield study | |
| Level 5 – Expert Opinion and Bench Research | Level 5.a – Systematic review of expert opinion |
| Level 5.b – Expert consensus | |
| Level 5.c – Bench research/single expert opinion | |
Table C.3.
| Levels of Evidence – Prognosis | |
|---|---|
| Level 1 – Inception Cohort Studies | Level 1.a – Systematic review of inception cohort studies |
| Level 1.b – Inception cohort study | |
| Level 2 – Studies of All or None | Level 2.a – Systematic review of all or none studies |
| Level 2.b – All or none studies | |
| Level 3 – Cohort Studies | Level 3.a – Systematic review of cohort studies (or control arm of RCT) |
| Level 3.b – Cohort study (or control arm of RCT) | |
| Level 4 – Case Series/Case Controlled/Historically Controlled Studies | Level 4.a – Systematic review of Case series/Case Controlled/Historically Controlled studies |
| Level 4.b – Individual Case series/Case Controlled/Historically Controlled study | |
| Level 5 – Expert Opinion and Bench Research | Level 5.a – Systematic review of expert opinion |
| Level 5.b – Expert consensus | |
| Level 5.c – Bench research/single expert opinion | |
Table C.4.
| Levels of Evidence – Economic Evaluations | |
|---|---|
| Level 1 | Decision model with assumptions and variables informed by systematic review and tailored to fit the decision-making context. |
| Level 2 | Systematic review of economic evaluations conducted in a setting similar to the decision makers. |
| Level 3 | Synthesis/review of economic evaluations undertaken in a setting similar to that in which the decision is to be made and which are of high quality (comprehensive and credible measurement of costs and health outcomes, sufficient time period covered, discounting, and sensitivity testing). |
| Level 4 | Economic evaluation of high quality (comprehensive and credible measurement of costs and health outcomes, sufficient time period covered, discounting and sensitivity testing) and conducted in setting similar to the decision-making context. |
| Level 5 | Synthesis/review of economic evaluations of moderate and/or poor quality (insufficient coverage of costs and health effects, no discounting, no sensitivity testing, time period covered insufficient). |
| Level 6 | Single economic evaluation of moderate or poor quality (see directly above level 5 description of studies). |
| Level 7 | Expert opinion on incremental cost effectives of intervention and comparator. |
Table C.5.
| Levels of Evidence – Meaningfulness | |
|---|---|
| Level 1 | Qualitative or mixed-methods systematic review |
| Level 2 | Qualitative or mixed-methods synthesis |
| Level 3 | Single qualitative study |
| Level 4 | Systematic review of expert opinion |
| Level 5 | Expert opinion |
REFERENCES
- 1.Herman TF, Bordoni B. Wound Classification. Wound Classification [Internet]. StatPearls. 2024; [cited 2024, July 5]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK554456/ [Google Scholar]
- 2.Wallace HA BB, Zito PM. Wound Healing Phases [Internet]. StatPearls. 2023; [cited 2024, July 5]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470443/ [Google Scholar]
- 3.Stroncek JD, Reichert WM. Overview of wound healing in different tissue types. In: Reichert WM, editor.. Indwelling neural implants: Strategies for contending with the in Vivo Environment. Boca Raton (FL): CRC Press/Taylor & Francis; 2008. Chapter 1. Available from: https://www.ncbi.nlm.nih.gov/books/NBK3938/ [Google Scholar]
- 4.Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89(3):219–29. DOI: 10.1177/0022034509359125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armstrong DG, Meyr AJ. Risk factors for impaired wound healing and wound complications. Wolters Kluwer; 2023. [Available from: https://www.uptodate.com/contents/risk-factors-for-impaired-wound-healing-and-wound-complications [Google Scholar]
- 6.Kumar D, Singh S, Shantanu K, Goyal R, Kushwaha NS, Gupta AK, et al. Need of revision of lower limb amputations in a north Indian tertiary care centre. J Clin Diagn Res. 2015;9(12):Rc01–3. DOI: 10.7860/jcdr/2015/16385.6886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choo YJ, Kim DH, Chang MC. Amputation stump management: A narrative review. World J Clin Cases. 2022;10(13):3981–8. DOI: 10.12998/wjcc.v10.i13.3981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wound management clinical practice guidelines tissue viability service [Internet]. Trust ELNF. 2019; [cited 2024, July 5]. Available from: https://www.elft.nhs.uk/sites/default/files/Wound%20Management%20Guidelines%206.0.pdf [Google Scholar]
- 9.Black JM, Brindle CT, Honaker JS. Differential diagnosis of suspected deep tissue injury. Int Wound J. 2016;13(4):531–9. DOI: 10.1111/iwj.12471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peart J. The aetiology of deep tissue injury: A literature review. Br J Nurs. 2016;25(15):840–3. DOI: 10.12968/bjon.2016.25.15.840 [DOI] [PubMed] [Google Scholar]
- 11.Johannesson A, Larsson GU, Oberg T, Atroshi I. Comparison of vacuum-formed removable rigid dressing with conventional rigid dressing after transtibial amputation: Similar outcome in a randomized controlled trial involving 27 patients. Acta Orthop. 2008;79(3):361–9. DOI: 10.1080/17453670710015265 [DOI] [PubMed] [Google Scholar]
- 12.Miller TA, Paul R, Forthofer M, Wurdeman SR. Impact of time to receipt of prosthesis on total healthcare costs 12 months postamputation. Am J Phys Med Rehabil. 2020;99(11):1026–31. DOI: 10.1097/phm.0000000000001473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geertzen JH, Martina JD, Rietman HS. Lower limb amputation. Part 2: Rehabilitation—a 10 year literature review. Prosthet Orthot Int. 2001;25(1):14–20. DOI: 10.1080/03093640108726563 [DOI] [PubMed] [Google Scholar]
- 14.Singh RK, Prasad G. Long-term mortality after lower-limb amputation. Prosthet Orthot Int. 2016;40(5):545–51. DOI: 10.1177/0309364615596067 [DOI] [PubMed] [Google Scholar]
- 15.Pran L, Harnanan D, Baijoo S, Short A, Cave C, Maharaj R, et al. Major lower limb amputations: Recognizing pitfalls. Cureus. 2021;13(8):e16972. DOI: 10.7759/cureus.16972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Day JD, Dionne CP, James S, Wang H. Determinants of healing and readiness for prosthetic fitting after transtibial amputation: Integrative literature review. Prosthet Orthot Int. 2023;47(1):43–53. DOI: 10.1097/pxr.0000000000000163 [DOI] [PubMed] [Google Scholar]
- 17.Optimising the timing for prosthetic fitting [Internet]. Bush & Co. 2024; [cited 2024, July 5]. Available from: https://www.bushco.co.uk/news/optimal-time-for-fitting-a-prosthetic.html [Google Scholar]
- 18.Turner S, Belsi A, McGregor AH. Issues faced by prosthetists and physiotherapists during lower-limb prosthetic rehabilitation: A thematic analysis. Front Rehabil Sci. 2021;2:795021. DOI: 10.3389/fresc.2021.795021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molina CA, Faulk J. Lower extremity amputation [Internet]. StatPearls Publishing. 2022; [cited 2024, July 5]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK546594/ [PubMed] [Google Scholar]
- 20.Spampinato SF, Caruso GI, De Pasquale R, Sortino MA, Merlo S. The treatment of impaired wound healing in diabetes: Looking among old drugs. Pharmaceuticals (Basel). 2020;13(4). DOI: 10.3390/ph13040060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Focus Area: Biomarkers [Internet]. FDA (U.S. Food and Drug Administration). 2022; [cited 2024, July 5]. Available from: https://www.fda.gov/science-research/focus-areas-regulatory-science-report/focus-area-biomarkers [Google Scholar]
- 22.Lindley LE, Stojadinovic O, Pastar I, Tomic-Canic M. Biology and biomarkers for wound healing. Plast Reconstr Surg. 2016;138(3 Suppl):18s–28s. DOI: 10.1097/prs.0000000000002682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strimbu K, Tavel JA. What are biomarkers? Curr Opin HIV AIDS. 2010;5(6):463–6. DOI: 10.1097/COH.0b013e32833ed177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bramley JL, Worsley PR, Bader DL, Everitt C, Darekar A, King L, et al. Changes in tissue composition and load response after transtibial amputation indicate biomechanical adaptation. Ann Biomed Eng. 2021;49(12):3176–88. DOI: 10.1007/s10439-021-02858-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanders JE, Fatone S. Residual limb volume change: systematic review of measurement and management. J Rehabil Res Dev. 2011;48(8):949–86. DOI: 10.1682/jrrd.2010.09.0189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel S, Maheshwari A, Chandra A. Biomarkers for wound healing and their evaluation. J Wound Care. 2016;25(1):46–55. DOI: 10.12968/jowc.2016.25.1.46 [DOI] [PubMed] [Google Scholar]
- 27.Bhutda S, Surve MV, Anil A, Kamath K, Singh N, Modi D, et al. Histochemical Staining of Collagen and Identification of Its Subtypes by Picrosirius Red Dye in Mouse Reproductive Tissues. Bio Protoc. 2017;7(21):e2592. DOI: 10.21769/BioProtoc.2592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gould L, Li WW. Defining complete wound closure: Closing the gap in clinical trials and practice. Wound Repair Regen. 2019;27(3):201–24. DOI: 10.1111/wrr.12707 [DOI] [PubMed] [Google Scholar]
- 29.Hata Y, Iida O, Okamoto S, Ishihara T, Nanto K, Tsujumura T, et al. Additional risk stratification using local and systemic factors for patients with critical limb ischaemia undergoing endovascular therapy in the Wi-Fi era. Eur J Vasc Endovasc Surg. 2019;58(4):548–55. DOI: 10.1016/j.ejvs.2019.06.005 [DOI] [PubMed] [Google Scholar]
- 30.Furuyama T, Yamashita S, Yoshiya K, Kurose S, Yoshino S, Nakayama K, et al. The Controlling nutritional status score is significantly associated with complete ulcer healing in patients with critical limb ischemia. Ann Vasc Surg. 2020;66:510–7. DOI: 10.1016/j.avsg.2019.12.031 [DOI] [PubMed] [Google Scholar]
- 31.Hung SY, Tsai JS, Yeh JT, Chen KH, Lin CN, Yang HM, et al. Amino acids and wound healing in people with limb-threatening diabetic foot ulcers. J Diabetes Complications. 2019;33(10):107403. DOI: 10.1016/j.jdiacomp.2019.06.008 [DOI] [PubMed] [Google Scholar]
- 32.Furuyama T, Onohara T, Yamashita S, Yoshiga R, Yoshiya K, Inoue K, et al. Prognostic factors of ulcer healing and amputation-free survival in patients with critical limb ischemia. Vascular. 2018;26(6):626–33. DOI: 10.1177/1708538118786864 [DOI] [PubMed] [Google Scholar]
- 33.Leaper DJ, Durani P. Topical antimicrobial therapy of chronic wounds healing by secondary intention using iodine products. Int Wound J. 2008;5(2):361–8. DOI: 10.1111/j.1742-481X.2007.00406.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gosain A, DiPietro LA. Aging and wound healing. World J Surg. 2004;28(3):321–6. DOI: 10.1007/s00268-003-7397-6 [DOI] [PubMed] [Google Scholar]
- 35.Peters MDJ, Marnie C, Tricco AC, Pollock D, Munn Z, Alexander L, et al. Updated methodological guidance for the conduct of scoping reviews. JBI Evid Synth. 2020;18(10):2119–26. DOI: 10.11124/jbies-20-00167 [DOI] [PubMed] [Google Scholar]
- 36.Pollock D, Davies EL, Peters MDJ, Tricco AC, Alexander L, McInerney P, et al. Undertaking a scoping review: A practical guide for nursing and midwifery students, clinicians, researchers, and academics. J Adv Nurs. 2021;77(4):2102–13. DOI: 10.1111/jan.14743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pollock D, Peters MDJ, Khalil H, McInerney P, Alexander L, Tricco AC, et al. Recommendations for the extraction, analysis, and presentation of results in scoping reviews. JBI Evid Synth. 2023;21(3):520–32. DOI: 10.11124/jbies-22-00123 [DOI] [PubMed] [Google Scholar]
- 38.Khalil H, Peters MD, Tricco AC, Pollock D, Alexander L, McInerney P, et al. Conducting high quality scoping reviews-challenges and solutions. J Clin Epidemiol. 2021;130:156–60. DOI: 10.1016/j.jclinepi.2020.10.009 [DOI] [PubMed] [Google Scholar]
- 39.PRISMA for Scoping Reviews [Internet]. PRISMA. 2024; [cited 2024, July 5]. Available from: http://www.prisma-statement.org/Extensions/ScopingReviews?AspxAutoDetectCookieSupport=1 [Google Scholar]
- 40.Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann Intern Med. 2018;169(7):467–73. DOI: 10.7326/m18-0850 [DOI] [PubMed] [Google Scholar]
- 41.O'Brien KK, Colquhoun H, Levac D, Baxter L, Tricco AC, Straus S, et al. Advancing scoping study methodology: a web-based survey and consultation of perceptions on terminology, definition and methodological steps. BMC Health Serv Res. 2016;16:305. DOI: 10.1186/s12913-016-1579-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hollander JE, Singer AJ, Valentine S. Comparison of wound care practices in pediatric and adult lacerations repaired in the emergency department. Pediatr Emerg Care. 1998;14(1):15–8. DOI: 10.1097/00006565-199802000-00004 [DOI] [PubMed] [Google Scholar]
- 43.Lindaman LM. Bone healing in children. Clin Podiatr Med Surg. 2001;18(1):97–108. [PubMed] [Google Scholar]
- 44.Canêo LF, Neirotti R. The importance of the proper definition of adulthood: What is and what is not included in a scientific publication. Braz J Cardiovasc Surg. 2017;32(1):60. DOI: 10.21470/1678-9741-2016-0049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Family Law Reform Act 1969 [Internet]. UK Public General Acts. 1969; [cited 2024, July 5]. Available from: https://www.legislation.gov.uk/ukpga/1969/46/section/1 [Google Scholar]
- 46.Giesen LJ, van den Boom AL, van Rossem CC, den Hoed PT, Wijnhoven BP. Retrospective multicenter study on risk factors for surgical site infections after appendectomy for acute appendicitis. Dig Surg. 2017;34(2):103–7. DOI: 10.1159/000447647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Surgical site infections: Prevention and treatment [Internet]. NICE. 2020; [cited 2024, July 5]. Available from: https://www.nice.org.uk/guidance/ng125 [Google Scholar]
- 48.Marques R, Lopes M, Ramos P, Neves Amado J, Alves P. Prognostic factors for delayed healing of complex wounds in adults: A scoping review protocol. Nurs Rep. 2022;12(4):904–11. DOI: 10.3390/nursrep12040087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bryda EC. The Mighty Mouse: the impact of rodents on advances in biomedical research. Mo Med. 2013;110(3):207–11. [PMC free article] [PubMed] [Google Scholar]
- 50.Tierney E, O'Rourke C, Fenton JE. What is the role of ‘the letter to the editor’? Eur Arch Otorhinolaryngol. 2015;272(9):2089–93. DOI: 10.1007/s00405-014-3289-7 [DOI] [PubMed] [Google Scholar]
- 51.Prontosan for treating acute and chronic wounds [Internet]. NICE. 2022; [cited 2024, July 5]. Available from: https://www.nice.org.uk/guidance/mtg67 [Google Scholar]
- 52.Bekele F, Chelkeba L. Amputation rate of diabetic foot ulcer and associated factors in diabetes mellitus patients admitted to Nekemte referral hospital, western Ethiopia: Prospective observational study. J Foot Ankle Res. 2020;13(1):65. DOI: 10.1186/s13047-020-00433-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jenkins DA, Mohamed S, Taylor JK, Peek N, van der Veer SN. Potential prognostic factors for delayed healing of common, non-traumatic skin ulcers: A scoping review. Int Wound J. 2019;16(3):800–12. DOI: 10.1111/iwj.13100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Skopec M, Issa H, Reed J, Harris M. The role of geographic bias in knowledge diffusion: a systematic review and narrative synthesis. Res Integr Peer Rev. 2020;5:2. DOI: 10.1186/s41073-019-0088-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mulimani P. Publication bias towards Western populations harms humanity. Nat Hum Behav. 2019;3(10):1026–7. DOI: 10.1038/s41562-019-0720-5 [DOI] [PubMed] [Google Scholar]
- 56.Gao H, Yous ML, Connelly D, Hung L, Garnett A, Hay ME, et al. Virtual team-based care planning with older persons in formal care settings: A scoping review protocol. BMJ Open. 2021;11(11):e054900. DOI: 10.1136/bmjopen-2021-054900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pham MT, Rajić A, Greig JD, Sargeant JM, Papadopoulos A, McEwen SA. A scoping review of scoping reviews: Advancing the approach and enhancing the consistency. Res Synth Methods. 2014;5(4):371–85. DOI: 10.1002/jrsm.1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ut TB, Peta Ellen T, Michelle B-J, Keryln C, Emily H, Peter AL, et al. A scoping review of research in chronic wounds: Protocol. Wound Practice and Research. 2021;29(4). DOI: 10.33235/wpr.29.4.234-237 [DOI] [Google Scholar]
- 59.Simonsen T, Sturge J, Duff C. Healing architecture in healthcare: A scoping review. Herd. 2022;15(3):315–28. DOI: 10.1177/19375867211072513 [DOI] [PubMed] [Google Scholar]
- 60.Hanneke R, Asada Y, Lieberman L, Neubauer L, & Fagen M. The scoping review method: Mapping the literature in “structural change” public health interventions. In Sage Research Methods Cases Part 2. SAGE Publications, Ltd., 2017. 10.4135/9781473999008 [DOI] [Google Scholar]
- 61.Kmet Leanne M., Lee Robert C., Cook LS. Standard quality assessment criteria for evaluating primary research papers from a variety of fields [Internet]. AHFMR. 2014; [cited 2024, July 5]. Available from: https://www.ihe.ca/download/standard_quality_assessment_criteria_for_evaluating_primary_research_papers_from_a_variety_of_fields.pdf [Google Scholar]
- 62.Maharaj S, Harding R. The needs, models of care, interventions and outcomes of palliative care in the Caribbean: A systematic review of the evidence. BMC Palliat Care. 2016;15:9. DOI: 10.1186/s12904-016-0079-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dewan P. Words versus pictures: Leveraging the research on visual communication. Partnership: The Canadian Journal of Library and Information Practice and Research. 2015;10. DOI: 10.21083/partnership.v10i1.3137 [DOI]
- 64.Williams-Reid H, Buis A, Johannesson A, Lechler K. Wound management, healing, and early prosthetic rehabilitation: a scoping review of biomarkers. 2023. Abstract from The Future of prosthetics and Orthotics- 2nd CDT P&O conference, Glasgow, United Kingdom. [Google Scholar]
- 65.Abedin-Do A, Zhang Z, Douville Y, Méthot M, Bernatchez J, Rouabhia M. Engineering diabetic human skin equivalent for in vitro and in vivo applications. Front Bioeng Biotechnol. 2022;10:989888. DOI: 10.3389/fbioe.2022.989888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Abedin-Do A, Zhang Z, Douville Y, Méthot M, Rouabhia M. Effect of electrical stimulation on diabetic human skin fibroblast growth and the secretion of cytokines and growth factors involved in wound healing. Biology (Basel). 2021;10(7). DOI: 10.3390/biology10070641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Al-Rikabi AHA, Tobin DJ, Riches-Suman K, Thornton MJ. Dermal fibroblasts cultured from donors with type 2 diabetes mellitus retain an epigenetic memory associated with poor wound healing responses. Sci Rep. 2021;11(1):1474. DOI: 10.1038/s41598-020-80072-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Altay FA, Kuzi S, Altay M, Ateş İ, Gürbüz Y, Tütüncü EE, et al. Predicting diabetic foot ulcer infection using the neutrophil-to-lymphocyte ratio: a prospective study. J Wound Care. 2019;28(9):601–7. DOI: 10.12968/jowc.2019.28.9.601 [DOI] [PubMed] [Google Scholar]
- 69.Bachar-Wikstrom E, Manchanda M, Bansal R, Karlsson M, Kelly-Pettersson P, Sköldenberg O, et al. Endoplasmic reticulum stress in human chronic wound healing: Rescue by 4-phenylbutyrate. Int Wound J. 2021;18(1):49–61. DOI: 10.1111/iwj.13525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bai H, Kyu-Cheol N, Wang Z, Cui Y, Liu H, Liu H, et al. Regulation of inflammatory microenvironment using a self-healing hydrogel loaded with BM-MSCs for advanced wound healing in rat diabetic foot ulcers. J Tissue Eng. 2020;11:2041731420947242. DOI: 10.1177/2041731420947242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bayer A, Lammel J, Lippross S, Klüter T, Behrendt P, Tohidnezhad M, et al. Platelet-released growth factors induce psoriasin in keratinocytes: Implications for the cutaneous barrier. Ann Anat. 2017;213:25–32. DOI: 10.1016/j.aanat.2017.04.002 [DOI] [PubMed] [Google Scholar]
- 72.Brookes JDL, Jaya JS, Tran H, Vaska A, Werner-Gibbings K, D'Mello AC, et al. Broad-ranging nutritional deficiencies predict amputation in diabetic foot ulcers. Int J Low Extrem Wounds. 2020;19(1):27–33. DOI: 10.1177/1534734619876779 [DOI] [PubMed] [Google Scholar]
- 73.Chahrour MA, Kharroubi H, Al Tannir AH, Assi S, Habib JR, Hoballah JJ. Hypoalbuminemia is associated with mortality in patients undergoing lower extremity amputation. Ann Vasc Surg. 2021;77:138–45. DOI: 10.1016/j.avsg.2021.05.047 [DOI] [PubMed] [Google Scholar]
- 74.Ezeani IU, Ugwu ET, Adeleye FO, Gezawa ID, Okpe IO, Enamino MI. Determinants of wound healing in patients hospitalized for diabetic foot ulcer: results from the MEDFUN study. Endocr Regul. 2020;54(3):207–16. DOI: 10.2478/enr-2020-0023 [DOI] [PubMed] [Google Scholar]
- 75.Farooque U, Lohano AK, Hussain Rind S, Rind MS, Sr., Karimi S, Jaan A, et al. Correlation of hemoglobin A1C with wagner classification in patients with diabetic foot. Cureus. 2020;12(7):e9199. DOI: 10.7759/cureus.9199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Feldbrin Z, Omelchenko E, Lipkin A, Shargorodsky M. Osteopontin levels in plasma, muscles, and bone in patient with non-healing diabetic foot ulcers: A new player in wound healing process? J Diabetes Complications. 2018;32(8):795–8. DOI: 10.1016/j.jdiacomp.2018.05.009 [DOI] [PubMed] [Google Scholar]
- 77.Hohendorff J, Drozdz A, Borys S, Ludwig-Slomczynska AH, Kiec-Wilk B, Stepien EL, et al. Effects of negative pressure wound therapy on levels of angiopoetin-2 and other selected circulating signaling molecules in patients with diabetic foot ulcer. J Diabetes Res. 2019;2019:1756798. DOI: 10.1155/2019/1756798 [DOI] [PMC free article] [PubMed]
- 78.Icli B, Wu W, Ozdemir D, Li H, Haemmig S, Liu X, et al. MicroRNA-135a-3p regulates angiogenesis and tissue repair by targeting p38 signaling in endothelial cells. Faseb j. 2019;33(4):5599–614. DOI: 10.1096/fj.201802063RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jere SW, Houreld NN, Abrahamse H. Photobiomodulation activates the PI3K/AKT pathway in diabetic fibroblast cells in vitro. J Photochem Photobiol B. 2022;237:112590. DOI: 10.1016/j.jphotobiol.2022.112590 [DOI] [PubMed] [Google Scholar]
- 80.Laiva AL, O'Brien FJ, Keogh MB. SDF-1α Gene-activated collagen scaffold restores pro-angiogenic wound healing features in human diabetic adipose-derived stem cells. Biomedicines. 2021;9(2). DOI: 10.3390/biomedicines9020160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li G, Zou X, Zhu Y, Zhang J, Zhou L, Wang D, et al. Expression and influence of matrix metalloproteinase-9/tissue inhibitor of metalloproteinase-1 and vascular endothelial growth factor in diabetic foot ulcers. Int J Low Extrem Wounds. 2017;16(1):6–13. DOI: 10.1177/1534734617696728 [DOI] [PubMed] [Google Scholar]
- 82.Liu Y, Wang F, Chen B. Anti-Inflammatory and antioxidant effects of chrysin mitigates diabetic foot ulcers. Int J Pharmacol. 2023;19(1):122–30. DOI: 10.3923/ijp.2023.122.130 [DOI] [Google Scholar]
- 83.MacDonald A, Brodell JD, Jr., Daiss JL, Schwarz EM, Oh I. Evidence of differential microbiomes in healing versus non-healing diabetic foot ulcers prior to and following foot salvage therapy. J Orthop Res. 2019;37(7):1596–603. DOI: 10.1002/jor.24279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Metineren H, Dülgeroğlu TC. Comparison of the neutrophil/lymphocyte ratio and c-reactive protein levels in patients with amputation for diabetic foot ulcers. Int J Low Extrem Wounds. 2017;16(1):23–8. DOI: 10.1177/1534734617696729 [DOI] [PubMed] [Google Scholar]
- 85.Miller A, Jeyapalina S, Agarwal J, Mansel M, Beck JP. A preliminary, observational study using whole-blood RNA sequencing reveals differential expression of inflammatory and bone markers post-implantation of percutaneous osseointegrated prostheses. PLoS One. 2022;17(5):e0268977. DOI: 10.1371/journal.pone.0268977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Morey M, O'Gaora P, Pandit A, Hélary C. Hyperglycemia acts in synergy with hypoxia to maintain the pro-inflammatory phenotype of macrophages. PLoS One. 2019;14(8):e0220577. DOI: 10.1371/journal.pone.0220577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Neama NA, Darweesh M, Al-Obiadi AB. Prevalence and antibiotic susceptibility pattern in diabetic footulcer infection with Ev Alua Tion The Role Of biomarker Il-12 in disease. Biochem Cell Arch. 2018;18:2321–8. [Google Scholar]
- 88.Newhall KA, Bekelis K, Suckow BD, Gottlieb DJ, Farber AE, Goodney PP, et al. The relationship of regional hemoglobin A1c testing and amputation rate among patients with diabetes. Vascular. 2017;25(2):142–8. DOI: 10.1177/1708538116650099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Raţiu IA, Raţiu CA, Miclăuş V, Boşca AB, Turan Kazancioğlu R, Constantin AM, et al. The pioneer use of a modified PRGF-Endoret® technique for wound healing in a hemodialyzed diabetic patient in a terminal stage of renal disease. Rom J Morphol Embryol. 2021;62(2):465–73. DOI: 10.47162/rjme.62.2.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Reiner MM, Khoury WE, Canales MB, Chmielewski RA, Patel K, Razzante MC, et al. Procalcitonin as a biomarker for predicting amputation level in lower extremity infections. J Foot Ankle Surg. 2017;56(3):484–91. DOI: 10.1053/j.jfas.2017.01.014 [DOI] [PubMed] [Google Scholar]
- 91.Sapienza P, Mingoli A, Borrelli V, Brachini G, Biacchi D, Sterpetti AV, et al. Inflammatory biomarkers, vascular procedures of lower limbs, and wound healing. Int Wound J. 2019;16(3):716–23. DOI: 10.1111/iwj.13086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sawaya AP, Jozic I, Stone RC, Pastar I, Egger AN, Stojadinovic O, et al. Mevastatin promotes healing by targeting caveolin-1 to restore EGFR signaling. JCI Insight. 2019;4(23). DOI: 10.1172/jci.insight.129320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Seçkin MF, Özcan Ç, Çamur S, Polat Ö, Batar S. Predictive factors and amputation level for reamputation in patients with diabetic foot: A retrospective case-control study. J Foot Ankle Surg. 2022;61(1):43–7. DOI: 10.1053/j.jfas.2021.06.006 [DOI] [PubMed] [Google Scholar]
- 94.Sharma GR, Kumar V, Kanojia RK, Vaiphei K, Kansal R. Fast and slow myosin as markers of muscle regeneration in mangled extremities: a pilot study. Eur J Orthop Surg Traumatol. 2019;29(7):1539–47. DOI: 10.1007/s00590-019-02448-w [DOI] [PubMed] [Google Scholar]
- 95.Shen YY, Zhang RR, Liu QY, Li SY, Yi S. Robust temporal changes of cellular senescence and proliferation after sciatic nerve injury. Neural Regen Res. 2022;17(7):1588–95. DOI: 10.4103/1673-5374.330619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shetty S, Pavan G, Shetty A, Kumari S, Shetty P, Patil P. Molecular signatures in diabetic foot ulcer by integrated gene expression profiling via bioinformatics analysis. Biomedicine. 2022;42:713–9. DOI: 10.51248/.v42i4.1798 [DOI] [Google Scholar]
- 97.Simsir IY, Sengoz Coskun NS, Akcay YY, Cetinkalp S. The relationship between blood hypoxia-inducible factor-1α, fetuin-A, fibrinogen, homocysteine, and amputation level. Int J Low Extrem Wounds. 2022;21(4):405–13. DOI: 10.1177/1534734620948342 [DOI] [PubMed] [Google Scholar]
- 98.Spoer DL, Shin SE, Kim KG, Haffner ZK, Linnartz KS, Attinger CE, et al. Perioperative evaluation of nutritional status to predict complications in patients with major lower extremity amputation. Wounds. 2023;35(3):59–65. DOI: 10.25270/wnds/22070 [DOI] [PubMed] [Google Scholar]
- 99.Sun H, Yang C, Liu X, Liang J, Geng H. Effectiveness of negative pressure wound therapy of diabetic foot ulcers using Periplaneta Americana (Kangfuxin liquid) irrigation. Int J Low Extrem Wounds. 2023:15347346231176917. DOI: 10.1177/15347346231176917 [DOI] [PubMed]
- 100.Ugwu E, Adeleye O, Gezawa I, Okpe I, Enamino M, Ezeani I. Predictors of lower extremity amputation in patients with diabetic foot ulcer: findings from MEDFUN, a multi-center observational study. J Foot Ankle Res. 2019;12:34. DOI: 10.1186/s13047-019-0345-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Walsh SA, Davis TA. Key early proinflammatory signaling molecules encapsulated within circulating exosomes following traumatic injury. J Inflamm (Lond). 2022;19(1):6. DOI: 10.1186/s12950-022-00303-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang J, Tinney D, Grynyshyn M, Pickering JG, Power A, Dubois L, et al. Microcirculation surrounding end-stage human chronic skin wounds is associated with endoglin/CD146/ALK-1 expression, endothelial cell proliferation and an absence of p16(Ink4a). Wound Repair Regen. 2023;31(3):321–37. DOI: 10.1111/wrr.13081 [DOI] [PubMed] [Google Scholar]
- 103.Wang T, Fan L, Liu J, Tao Y, Li X, Wang X, et al. Negative pressure wound therapy promotes wound healing by inhibiting inflammation in diabetic foot wounds: A role for NOD1 receptor. Int J Low Extrem Wounds. 2022:15347346221131844. DOI: 10.1177/15347346221131844 [DOI] [PubMed]
- 104.Xu S, Wang Y, Hu Z, Ma L, Zhang F, Liu P. Effects of neutrophil-to-lymphocyte ratio, serum calcium, and serum albumin on prognosis in patients with diabetic foot. Int Wound J. 2022;20. DOI: 10.1111/iwj.14019 [DOI] [PMC free article] [PubMed]
- 105.Yang SL, Zhu LY, Han R, Sun LL, Dou JT. Effect of negative pressure wound therapy on cellular fibronectin and transforming growth factor-β1 expression in diabetic foot wounds. Foot Ankle Int. 2017;38(8):893–900. DOI: 10.1177/1071100717704940 [DOI] [PubMed] [Google Scholar]
- 106.Yu XT, Wang F, Ding JT, Cai B, Xing JJ, Guo GH, et al. Tandem mass tag-based serum proteomic profiling revealed diabetic foot ulcer pathogenesis and potential therapeutic targets. Bioengineered. 2022;13(2):3171–82. DOI: 10.1080/21655979.2022.2027173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yuniati R, Innelya I, Rachmawati A, Charlex HJM, Rahmatika A, Khrisna MB, et al. Application of topical sucralfate and topical platelet-rich plasma improves wound healing in diabetic ulcer Rats Wound Model. J Exp Pharmacol. 2021;13:797–806. DOI: 10.2147/jep.S296767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang M, Zhang R, Li X, Cao Y, Huang K, Ding J, et al. CD271 promotes STZ-induced diabetic wound healing and regulates epidermal stem cell survival in the presence of the pTrkA receptor. Cell Tissue Res. 2020;379(1):181–93. DOI: 10.1007/s00441-019-03125-4 [DOI] [PubMed] [Google Scholar]
- 109.Adams BE, Edlinger JP, Ritterman Weintraub ML, Pollard JD. Three-year morbidity and mortality rates after nontraumatic transmetatarsal amputation. J Foot Ankle Surg. 2018;57(5):967–71. DOI: 10.1053/j.jfas.2018.03.047 [DOI] [PubMed] [Google Scholar]
- 110.Aguirre A, Sharma K, Arora A, Humphries MD. Early ABI testing may decrease risk of amputation for patients with lower extremity ulcers. Ann Vasc Surg. 2022;79:65–71. DOI: 10.1016/j.avsg.2021.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ahn J, Raspovic KM, Liu GT, Lavery LA, La Fontaine J, Nakonezny PA, et al. Renal function as a predictor of early transmetatarsal amputation failure. Foot Ankle Spec. 2019;12(5):439–51. DOI: 10.1177/1938640018816371 [DOI] [PubMed] [Google Scholar]
- 112.Alfawaz A, Kotha VS, Nigam M, Bekeny JC, Black CK, Tefera E, et al. Popliteal artery patency is an indicator of ambulation and healing after below-knee amputation in vasculopaths. Vascular. 2022;30(4):708–14. DOI: 10.1177/17085381211026498 [DOI] [PubMed] [Google Scholar]
- 113.Aljarrah Q, Allouh MZ, Husein A, Al-Jarrah H, Hallak A, Bakkar S, et al. Transmetatarsal amputations in patients with diabetes mellitus: A contemporary analysis from an academic tertiary referral centre in a developing community. PLoS One. 2022;17(11):e0277117. DOI: 10.1371/journal.pone.0277117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Anguiano-Hernandez YM, Contreras-Mendez L, de Los Angeles Hernandez-Cueto M, Muand Oz-Medina JE, Santillan-Verde MA, Barbosa-Cabrera RE, et al. Modification of HIF-1α, NF-aκB, IGFBP-3, VEGF and adiponectin in diabetic foot ulcers treated with hyperbaric oxygen. Undersea Hyperb Med. 2019;46(1):35–44. [PubMed] [Google Scholar]
- 115.Ariyanti AD, Zhang J, Marcelina O, Nugrahaningrum DA, Wang G, Kasim V, et al. Salidroside-pretreated mesenchymal stem cells enhance diabetic wound healing by promoting paracrine function and survival of mesenchymal stem cells under hyperglycemia. Stem Cells Transl Med. 2019;8(4):404–14. DOI: 10.1002/sctm.18-0143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Barć P, Antkiewicz M, Śliwa B, Baczyńska D, Witkiewicz W, Skóra JP. Treatment of critical limb ischemia by pIRES/VEGF165/HGF administration. Ann Vasc Surg. 2019;60:346–54. DOI: 10.1016/j.avsg.2019.03.013 [DOI] [PubMed] [Google Scholar]
- 117.Begum F, Manandhar S, Kumar G, Keni R, Sankhe R, Gurram PC, et al. Dehydrozingerone promotes healing of diabetic foot ulcers: A molecular insight. J Cell Commun Signal. 2023;17(3):673–88. DOI: 10.1007/s12079-022-00703-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Berli MC, Wanivenhaus F, Kabelitz M, Götschi T, Böni T, Rancic Z, et al. Predictors for reoperation after lower limb amputation in patients with peripheral arterial disease. Vasa. 2019;48(5):419–24. DOI: 10.1024/0301-1526/a000796 [DOI] [PubMed] [Google Scholar]
- 119.Bian J, Bao L, Gao X, Wen X, Zhang Q, Huang J, et al. Bacteria-engineered porous sponge for hemostasis and vascularization. J Nanobiotechnology. 2022;20(1):47. DOI: 10.1186/s12951-022-01254-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bibi S, Ahmad F, Alam MR, Ansar M, Yeou KS, Wahedi HM. lapachol-induced upregulation of sirt1/sirt3 is linked with improved skin wound healing in alloxan-induced diabetic mice. Iran J Pharm Res. 2021;20(3):419–30. DOI: 10.22037/ijpr.2021.112722.13914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bramley JL, Worsley PR, Bostan LE, Bader DL, Dickinson AS. Establishing a measurement array to assess tissue tolerance during loading representative of prosthetic use. Med Eng Phys. 2020;78:39–47. DOI: 10.1016/j.medengphy.2020.01.011 [DOI] [PubMed] [Google Scholar]
- 122.Campitiello F, Mancone M, Cammarota M, D'Agostino A, Ricci G, Stellavato A, et al. Acellular dermal matrix used in diabetic foot ulcers: Clinical outcomes supported by biochemical and histological analyses. Int J Mol Sci. 2021;22(13). DOI: 10.3390/ijms22137085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chan AS, Montbriand J, Eisenberg N, Roche-Nagle G. Outcomes of minor amputations in patients with peripheral vascular disease over a 10-year period at a tertiary care institution. Vascular. 2019;27(1):8–18. DOI: 10.1177/1708538118797544 [DOI] [PubMed] [Google Scholar]
- 124.Chaudhary N, Huda F, Roshan R, Basu S, Rajput D, Singh SK. Lower limb amputation rates in patients with diabetes and an infected foot ulcer: A prospective observational study. Wound Manag Prev. 2021;67(7):22–30. [PubMed] [Google Scholar]
- 125.Chen L, Ma W, Covassin N, Chen D, Zha P, Wang C, et al. Association of sleep-disordered breathing and wound healing in patients with diabetic foot ulcers. J Clin Sleep Med. 2021;17(5):909–16. DOI: 10.5664/jcsm.9088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cheng P, Dong Y, Hu Z, Huang S, Cao X, Wang P, et al. Biomarker prediction of postoperative healing of diabetic foot ulcers: A retrospective observational study of serum albumin. Journal of Wound Ostomy & Continence Nursing. 2021;48(4):339–44. DOI: 10.1097/won.0000000000000780 [DOI] [PubMed] [Google Scholar]
- 127.Chowdary AR, Maerz T, Henn D, Hankenson KD, Pagani CA, Marini S, et al. Macrophage-mediated PDGF activation correlates with regenerative outcomes following musculoskeletal trauma. Ann Surg. 2023;278(2):e349–e59. DOI: 10.1097/sla.0000000000005704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Das SK, Yuan YF, Li MQ. Predictors of delayed wound healing after successful isolated below-the-knee endovascular intervention in patients with ischemic foot ulcers. J Vasc Surg. 2018;67(4):1181–90. DOI: 10.1016/j.jvs.2017.08.077 [DOI] [PubMed] [Google Scholar]
- 129.Ding Y, Cui L, Zhao Q, Zhang W, Sun H, Zheng L. Platelet-rich fibrin accelerates skin wound healing in diabetic mice. Ann Plast Surg. 2017;79(3):e15–e9. DOI: 10.1097/sap.0000000000001091 [DOI] [PubMed] [Google Scholar]
- 130.Dinoto E, Ferlito F, La Marca MA, Tortomasi G, Urso F, Evola S, et al. The role of early revascularization and biomarkers in the management of diabetic foot ulcers: A single center experience. Diagnostics (Basel). 2022;12(2). DOI: 10.3390/diagnostics12020538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Doulamis A, Doulamis N, Angeli A, Lazaris A, Luthman S, Jayapala M, et al. A non-invasive photonics-based device for monitoring of diabetic foot ulcers: Architectural/sensorial components & technical specifications. Inventions. 2021;6(2):27. [Google Scholar]
- 132.Dutra LMA, Melo MC, Moura MC, Leme LAP, De Carvalho MR, Mascarenhas AN, et al. Prognosis of the outcome of severe diabetic foot ulcers with multidisciplinary care. J Multidiscip Healthc. 2019;12:349–59. DOI: 10.2147/jmdh.S194969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.El-Gizawy SA, Nouh A, Saber S, Kira AY. Deferoxamine-loaded transfersomes accelerates healing of pressure ulcers in streptozotocin-induced diabetic rats. J Drug Deliv Sci Technol. 2020;58:101732. DOI: 10.1016/j.jddst.2020.101732 [DOI] [Google Scholar]
- 134.Elliott CG, Wang J, Walker JT, Michelsons S, Dunmore-Buyze J, Drangova M, et al. Periostin and CCN2 scaffolds promote the wound healing response in the skin of diabetic mice. Tissue Eng Part A. 2019;25(17-18):1326–39. DOI: 10.1089/ten.TEA.2018.0268 [DOI] [PubMed] [Google Scholar]
- 135.Escuin-Ordinas H, Liu Y, Sun L, Hugo W, Dimatteo R, Huang RR, et al. Wound healing with topical BRAF inhibitor therapy in a diabetic model suggests tissue regenerative effects. PLoS One. 2021;16(6):e0252597. DOI: 10.1371/journal.pone.0252597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ferroni L, Gardin C, De Pieri A, Sambataro M, Seganfreddo E, Goretti C, et al. Treatment of diabetic foot ulcers with Therapeutic Magnetic Resonance (TMR®) improves the quality of granulation tissue. Eur J Histochem. 2017;61(3):2800. DOI: 10.4081/ejh.2017.2800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Friedman A, Siewe N. Mathematical model of chronic dermal wounds in diabetes and obesity. Bull Math Biol. 2020;82(10):137. DOI: 10.1007/s11538-020-00815-x [DOI] [PubMed] [Google Scholar]
- 138.Furuyama T, Yamashita S, Yoshiya K, Kurose S, Yoshino S, Nakayama K, et al. The controlling nutritional status score is significantly associated with complete ulcer healing in patients with critical limb ischemia. Ann Vasc Surg. 2020;66:510–7. DOI: 10.1016/j.avsg.2019.12.031 [DOI] [PubMed] [Google Scholar]
- 139.Gao C, Yang L, Ju J, Gao Y, Zhang K, Wu M, et al. Risk and prognostic factors of replantation failure in patients with severe traumatic major limb mutilation. Eur J Trauma Emerg Surg. 2022;48(4):3203–10. DOI: 10.1007/s00068-021-01876-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gao R, Zhou P, Li Y, Li Q. High glucose-induced IL-7/IL-7R upregulation of dermal fibroblasts inhibits angiogenesis in a paracrine way in delayed diabetic wound healing. J Cell Commun Signal. 2023;17(3):1023–38. DOI: 10.1007/s12079-023-00754-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gazzaruso C, Gallotti P, Pujia A, Montalcini T, Giustina A, Coppola A. Predictors of healing, ulcer recurrence and persistence, amputation and mortality in type 2 diabetic patients with diabetic foot: a 10-year retrospective cohort study. Endocrine. 2021;71(1):59–68. DOI: 10.1007/s12020-020-02431-0 [DOI] [PubMed] [Google Scholar]
- 142.Gülcü A, Etli M, Karahan O, Aslan A. Analysis of routine blood markers for predicting amputation/re-amputation risk in diabetic foot. Int Wound J. 2020;17(6):1996–2004. DOI: 10.1111/iwj.13491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Guo Z, Yue C, Qian Q, He H, Mo Z. Factors associated with lower-extremity amputation in patients with diabetic foot ulcers in a Chinese tertiary care hospital. Int Wound J. 2019;16(6):1304–13. DOI: 10.1111/iwj.13190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hansen RL, Langdahl BL, Jørgensen PH, Petersen KK, Søballe K, Stilling M. Changes in periprosthetic bone mineral density and bone turnover markers after osseointegrated implant surgery: A cohort study of 20 transfemoral amputees with 30-month follow-up. Prosthet Orthot Int. 2019;43(5):508–18. DOI: 10.1177/0309364619866599 [DOI] [PubMed] [Google Scholar]
- 145.He FL, Qiu S, Zou JL, Gu FB, Yao Z, Tu ZH, et al. Covering the proximal nerve stump with chondroitin sulfate proteoglycans prevents traumatic painful neuroma formation by blocking axon regeneration after neurotomy in Sprague Dawley rats. J Neurosurg. 2021;134(5):1599–609. DOI: 10.3171/2020.3.Jns193202 [DOI] [PubMed] [Google Scholar]
- 146.He S, Walimbe T, Chen H, Gao K, Kumar P, Wei Y, et al. Bioactive extracellular matrix scaffolds engineered with proangiogenic proteoglycan mimetics and loaded with endothelial progenitor cells promote neovascularization and diabetic wound healing. Bioact Mater. 2022;10:460–73. DOI: 10.1016/j.bioactmat.2021.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Huon JF, Gaborit B, Caillon J, Boutoille D, Navas D. A murine model of Staphylococcus aureus infected chronic diabetic wound: A new tool to develop alternative therapeutics. Wound Repair Regen. 2020;28(3):400–8. DOI: 10.1111/wrr.12802 [DOI] [PubMed] [Google Scholar]
- 148.Husakova J, Bem R, Fejfarova V, Jirkovska A, Woskova V, Jarosikova R, et al. Factors influencing the risk of major amputation in patients with diabetic foot ulcers treated by autologous cell therapy. J Diabetes Res. 2022;2022:3954740. DOI: 10.1155/2022/3954740 [DOI] [PMC free article] [PubMed]
- 149.Jeon BJ, Choi HJ, Kang JS, Tak MS, Park ES. Comparison of five systems of classification of diabetic foot ulcers and predictive factors for amputation. Int Wound J. 2017;14(3):537–45. DOI: 10.1111/iwj.12642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ji X, Jin P, Yu P, Wang P. Autophagy ameliorates Pseudomonas aeruginosa-infected diabetic wounds by regulating the toll-like receptor 4/myeloid differentiation factor 88 pathway. Wound Repair Regen. 2023;31(3):305–20. DOI: 10.1111/wrr.13074 [DOI] [PubMed] [Google Scholar]
- 151.Junaidi F, Muradi A, Pratama D, Suhartono R, Kekalih A. Effectiveness of doppler ultrasonography as a predictor of wound healing after below-knee amputation for peripheral arterial disease. Chirurgia (Bucur). 2020;115(5):618–25. DOI: 10.21614/chirurgia.115.5.618 [DOI] [PubMed] [Google Scholar]
- 152.Kasowanjete P, Abrahamse H, Houreld NN. Photobiomodulation at 660 nm stimulates in vitro diabetic wound healing via the Ras/MAPK pathway. Cells. 2023;12(7). DOI: 10.3390/cells12071080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Katagiri T, Kondo K, Shibata R, Hayashida R, Shintani S, Yamaguchi S, et al. Therapeutic angiogenesis using autologous adipose-derived regenerative cells in patients with critical limb ischaemia in Japan: A clinical pilot study. Sci Rep. 2020;10(1):16045. DOI: 10.1038/s41598-020-73096-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kee KK, Nair HKR, Yuen NP. Risk factor analysis on the healing time and infection rate of diabetic foot ulcers in a referral wound care clinic. J Wound Care. 2019;28(Sup1):S4–s13. DOI: 10.12968/jowc.2019.28.Sup1.S4 [DOI] [PubMed] [Google Scholar]
- 155.Kim BE, Goleva E, Hall CF, Park SH, Lee UH, Brauweiler AM, et al. Skin wound healing is accelerated by a lipid mixture representing major lipid components of chamaecyparis obtusa plant extract. J Invest Dermatol. 2018;138(5):1176–86. DOI: 10.1016/j.jid.2017.11.039 [DOI] [PubMed] [Google Scholar]
- 156.Kim KG, Mishu M, Zolper EG, Bhardwaj P, Rogers A, Dekker PK, et al. Nutritional markers for predicting lower extremity free tissue transfer outcomes in the chronic wound population. Microsurgery. 2023;43(1):51–6. DOI: 10.1002/micr.30794 [DOI] [PubMed] [Google Scholar]
- 157.Kim S, Piao J, Hwang DY, Park JS, Son Y, Hong HS. Substance P accelerates wound repair by promoting neovascularization and preventing inflammation in an ischemia mouse model. Life Sci. 2019;225:98–106. DOI: 10.1016/j.lfs.2019.04.015 [DOI] [PubMed] [Google Scholar]
- 158.Kimura T, Watanabe Y, Tokuoka S, Nagashima F, Ebisudani S, Inagawa K. Utility of skin perfusion pressure values with the society for vascular surgery wound, ischemia, and foot infection classification system. J Vasc Surg. 2019;70(4):1308–17. DOI: 10.1016/j.jvs.2019.01.045 [DOI] [PubMed] [Google Scholar]
- 159.Kodama A, Komori K, Koyama A, Sato T, Ikeda S, Tsuruoka T, et al. Impact of serum zinc level and oral zinc supplementation on clinical outcomes in patients undergoing infrainguinal bypass for chronic limb-threatening ischemia. Circ J. 2022;86(6):995–1006. DOI: 10.1253/circj.CJ-21-0832 [DOI] [PubMed] [Google Scholar]
- 160.Kolumam G, Wu X, Lee WP, Hackney JA, Zavala-Solorio J, Gandham V, et al. IL-22R ligands IL-20, IL-22, and IL-24 promote wound healing in diabetic db/db Mice. PLoS One. 2017;12(1):e0170639 DOI: 10.1371/journal.pone.0170639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Koyama A, Kodama A, Tsuruoka T, Fujii T, Sugimoto M, Banno H, et al. Zinc deficiency and clinical outcome after infrainguinal bypass grafting for critical limb ischemia. Circ Rep. 2020;2(3):167–73. DOI: 10.1253/circrep.CR-20-0003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kurkipuro J, Mierau I, Wirth T, Samaranayake H, Smith W, Kärkkäinen HR, et al. Four in one-combination therapy using live lactococcus lactis expressing three therapeutic proteins for the treatment of chronic non-healing wounds. PLoS One. 2022;17(2):e0264775. DOI: 10.1371/journal.pone.0264775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lee JV, Engel C, Tay S, DeSilva G, Desai K, Cashin J, et al. Impact of N-acetyl-cysteine on ischemic stumps following major lower extremity amputation: a pilot randomized clinical trial. Ann Surg. 2022;276(5):e302–e10. DOI: 10.1097/sla.0000000000005389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lee Y-H, Lin S-J. Chitosan/PVA Hetero-composite hydrogel containing antimicrobials, perfluorocarbon nanoemulsions, and growth factor-loaded nanoparticles as a multifunctional dressing for diabetic wound healing: synthesis, characterization, and in vitro/in vivo evaluation. Pharmaceutics. 2022;14(3):537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lee YH, Hong YL, Wu TL. Novel silver and nanoparticle-encapsulated growth factor co-loaded chitosan composite hydrogel with sustained antimicrobility and promoted biological properties for diabetic wound healing. Mater Sci Eng C Mater Biol Appl. 2021;118:111385. DOI: 10.1016/j.msec.2020.111385 [DOI] [PubMed] [Google Scholar]
- 166.Lee YJ, Ahn CM, Ko YG, Park KH, Lee JW, Lee SJ, et al. Skin perfusion pressure predicts early wound healing after endovascular therapy in chronic limb threatening ischaemia. Eur J Vasc Endovasc Surg. 2021;62(6):909–17. DOI: 10.1016/j.ejvs.2021.08.030 [DOI] [PubMed] [Google Scholar]
- 167.Leu JG, Chiang MH, Chen CY, Lin JT, Chen HM, Chen YL, et al. Adenine accelerated the diabetic wound healing by PPAR delta and angiogenic regulation. Eur J Pharmacol. 2018;818:569–77. DOI: 10.1016/j.ejphar.2017.11.027 [DOI] [PubMed] [Google Scholar]
- 168.Li B, Zhou Y, Chen J, Wang T, Li Z, Fu Y, et al. Long non-coding RNA H19 contributes to wound healing of diabetic foot ulcer. J Mol Endocrinol. 2020. DOI: 10.1530/jme-19-0242 [DOI] [PubMed]
- 169.Li C, Liu SY, Zhou LP, Min TT, Zhang M, Pi W, et al. Polydopamine-modified chitin conduits with sustained release of bioactive peptides enhance peripheral nerve regeneration in rats. Neural Regen Res. 2022;17(11):2544–50. DOI: 10.4103/1673-5374.339006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Li G, Li D, Wu C, Li S, Chen F, Li P, et al. Homocysteine-targeting compounds as a new treatment strategy for diabetic wounds via inhibition of the histone methyltransferase SET7/9. Exp Mol Med. 2022;54(7):988–98. DOI: 10.1038/s12276-022-00804-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Li J, Arora S, Ikeoka K, Smith J, Dash S, Kimura S, et al. The utility of geriatric nutritional risk index to predict outcomes in chronic limb-threatening ischemia. Catheter Cardiovasc Interv. 2022;99(1):121–33. DOI: 10.1002/ccd.29949 [DOI] [PubMed] [Google Scholar]
- 172.Li J, Chou H, Li L, Li H, Cui Z. Wound healing activity of neferine in experimental diabetic rats through the inhibition of inflammatory cytokines and nrf-2 pathway. Artif Cells Nanomed Biotechnol. 2020;48(1):96–106. DOI: 10.1080/21691401.2019.1699814 [DOI] [PubMed] [Google Scholar]
- 173.Li M, Li X, Gao Y, Yang Y, Yi C, Huang W, et al. Composite nanofibrous dressing loaded with Prussian blue and heparin for anti-inflammation therapy and diabetic wound healing. Int J Biol Macromol. 2023;242(Pt 3):125144. DOI: 10.1016/j.ijbiomac.2023.125144 [DOI] [PubMed] [Google Scholar]
- 174.Li S, Wang X, Chen J, Guo J, Yuan M, Wan G, et al. Calcium ion cross-linked sodium alginate hydrogels containing deferoxamine and copper nanoparticles for diabetic wound healing. Int J Biol Macromol. 2022;202:657–70. DOI: 10.1016/j.ijbiomac.2022.01.080 [DOI] [PubMed] [Google Scholar]
- 175.Lin BS, Chang CC, Tseng YH, Li JR, Peng YS, Huang YK. Using wireless near-infrared spectroscopy to predict wound prognosis in diabetic foot ulcers. Adv Skin Wound Care. 2020;33(1):1–12. DOI: 10.1097/01.ASW.0000613552.50065.d5 [DOI] [PubMed] [Google Scholar]
- 176.Lin DS, Lee JK. Mobile Health-Based Thermometer for monitoring wound healing after endovascular therapy in patients with chronic foot ulcer: Prospective cohort study. JMIR Mhealth Uhealth. 2021;9(5):e26468. DOI: 10.2196/26468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Liu Y, Zhang X, Yang L, Zhou S, Li Y, Shen Y, et al. Proteomics and transcriptomics explore the effect of mixture of herbal extract on diabetic wound healing process. Phytomedicine. 2023;116:154892. DOI: 10.1016/j.phymed.2023.154892 [DOI] [PubMed] [Google Scholar]
- 178.Luan H, Huiru G, Mo Z, Ren W, Guo H, Chu Z, et al. The bone alterations in hind limb amputation rats in vivo. Med Nov Technol Devices. 2020;8:100046. DOI: 10.1016/j.medntd.2020.100046 [DOI] [Google Scholar]
- 179.Majumdar M, Lella S, Hall RP, Sumetsky N, Waller HD, McElroy I, et al. Utilization of thromboelastography with platelet mapping to predict infection and poor wound healing in postoperative vascular patients. Ann Vasc Surg. 2022;87:213–24. DOI: 10.1016/j.avsg.2022.03.008 [DOI] [PubMed] [Google Scholar]
- 180.Manso G, Elias-Oliveira J, Guimarães JB, Pereira Í S, Rodrigues VF, Burger B, et al. Xenogeneic mesenchymal stem cell biocurative improves skin wounds healing in diabetic mice by increasing mast cells and the regenerative profile. Regen Ther. 2023;22:79–89. DOI: 10.1016/j.reth.2022.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.McLaughlin PJ, Cain JD, Titunick MB, Sassani JW, Zagon IS. Topical naltrexone is a safe and effective alternative to standard treatment of diabetic wounds. Adv Wound Care (New Rochelle). 2017;6(9):279–88. DOI: 10.1089/wound.2016.0725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Mehrvar S, Rymut KT, Foomani FH, Mostaghimi S, Eells JT, Ranji M, et al. Fluorescence imaging of mitochondrial redox state to assess diabetic wounds. IEEE J Transl Eng Health Med. 2019;7:1800809. DOI: 10.1109/jtehm.2019.2945323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mendoza-Marí Y, García-Ojalvo A, Fernández-Mayola M, Rodríguez-Rodríguez N, Martinez-Jimenez I, Berlanga-Acosta J. Epidermal growth factor effect on lipopolysaccharide-induced inflammation in fibroblasts derived from diabetic foot ulcer. Scars Burn Heal. 2022;8:20595131211067380. DOI: 10.1177/20595131211067380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Metcalf DG, Haalboom M, Bowler PG, Gamerith C, Sigl E, Heinzle A, et al. Elevated wound fluid pH correlates with increased risk of wound infection. Wound Med. 2019;26(1):100166. DOI: 10.1016/j.wndm.2019.100166. [DOI] [Google Scholar]
- 185.Modaghegh MHS, Saberianpour S, Amoueian S, Shahri JJ, Rahimi H. The effect of redox signaling on extracellular matrix changes in diabetic wounds leading to amputation. Biochem Biophys Rep. 2021;26:101025. DOI: 10.1016/j.bbrep.2021.101025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Mokoena DR, Houreld NN, Dhilip Kumar SS, Abrahamse H. Photobiomodulation at 660 nm stimulates fibroblast differentiation. Lasers Surg Med. 2020;52(7):671–81. DOI: 10.1002/lsm.23204 [DOI] [PubMed] [Google Scholar]
- 187.Moon KC, Kim KB, Han SK, Jeong SH, Dhong ES. Risk factors for major amputation on hindfoot ulcers in hospitalized diabetic patients. Adv Wound Care (New Rochelle). 2019;8(5):177–85. DOI: 10.1089/wound.2018.0814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Moon KC, Kim SB, Han SK, Jeong SH, Dhong ES. Risk factors for major amputation in hospitalized diabetic patients with forefoot ulcers. Diabetes Res Clin Pract. 2019;158:107905. DOI: 10.1016/j.diabres.2019.107905 [DOI] [PubMed] [Google Scholar]
- 189.Mutlu HS, Erdoğan A, Tapul L. Autologously transplanted dermal fibroblasts improved diabetic wound in rat model. Acta Histochemica. 2020;122(5):151552. DOI: 10.1016/j.acthis.2020.151552 [DOI] [PubMed] [Google Scholar]
- 190.Nasrullah MZ. Caffeic acid phenethyl ester loaded PEG-PLGA nanoparticles enhance wound healing in diabetic rats. Antioxidants (Basel). 2022;12(1). DOI: 10.3390/antiox12010060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Nayak M, Nag HL, Nag TC, Digge V, Yadav R. Ultrastructural and histological changes in tibial remnant of ruptured anterior cruciate ligament stumps: a transmission electron microscopy and immunochemistry-based observational study. Musculoskelet Surg. 2020;104(1):67–74. DOI: 10.1007/s12306-019-00599-x [DOI] [PubMed] [Google Scholar]
- 192.Nensat C, Songjang W, Tohtong R, Suthiphongchai T, Phimsen S, Rattanasinganchan P, et al. Porcine placenta extract improves high-glucose-induced angiogenesis impairment. BMC Complement Med Ther. 2021;21(1):66. DOI: 10.1186/s12906-021-03243-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Nishikai-Yan Shen T, Kado M, Hagiwara H, Fujimura S, Mizuno H, Tanaka R. MMP9 secreted from mononuclear cell quality and quantity culture mediates STAT3 phosphorylation and fibroblast migration in wounds. Regen Ther. 2021;18:464–71. DOI: 10.1016/j.reth.2021.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Norvell DC, Czerniecki JM. Risks and risk factors for ipsilateral re-amputation in the first year following first major unilateral dysvascular amputation. Eur J Vasc Endovasc Surg. 2020;60(4):614–21. DOI: 10.1016/j.ejvs.2020.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Nystrom LM, Mesko NW, Jin Y, Shah C, Spiguel A, White J, et al. Transcutaneous oximetry does not reliably predict wound-healing complications in preoperatively radiated soft tissue sarcoma. Clin Orthop Relat Res. 2023;481(3):542–9. DOI: 10.1097/corr.0000000000002279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ou S, Xu C, Yang Y, Chen Y, Li W, Lu H, et al. Transverse tibial bone transport enhances distraction osteogenesis and vascularization in the treatment of diabetic foot. Orthop Surg. 2022;14(9):2170–9. DOI: 10.1111/os.13416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Pan X, You C, Chen G, Shao H, Han C, Zhi L. Skin perfusion pressure for the prediction of wound healing in critical limb ischemia: A meta-analysis. Arch Med Sci. 2018;14(3):481–7. DOI: 10.5114/aoms.2016.62220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Rajagopalan C, Viswanathan V, Rajsekar S, Selvaraj B, Daniel L. Diabetic foot ulcers—comparison of performance of ankle-brachial index and transcutaneous partial oxygen pressure in predicting outcome. Int J Diabetes Dev Ctries. 2018;38(2):179–84. DOI: 10.1007/s13410-017-0580-3 [DOI] [Google Scholar]
- 199.Razjouyan J, Grewal GS, Talal TK, Armstrong DG, Mills JL, Najafi B. Does physiological stress slow down wound healing in patients with diabetes? J Diabetes Sci Technol. 2017;11(4):685–92. DOI: 10.1177/1932296817705397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ridiandries A, Bursill C, Tan J. Broad-spectrum inhibition of the cc-chemokine class improves wound healing and wound angiogenesis. Int J Mol Sci. 2017;18(1). DOI: 10.3390/ijms18010155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Salaun P, Desormais I, Lapébie FX, Rivière AB, Aboyans V, Lacroix P, et al. Comparison of ankle pressure, systolic toe pressure, and transcutaneous oxygen pressure to predict major amputation after 1 year in the COPART Cohort. Angiology. 2019;70(3):229–36. DOI: 10.1177/0003319718793566 [DOI] [PubMed] [Google Scholar]
- 202.Senturk B, Demircan BM, Ozkan AD, Tohumeken S, Delibasi T, Guler MO, et al. Diabetic wound regeneration using heparin-mimetic peptide amphiphile gel in db/db mice. Biomater Sci. 2017;5(7):1293–303. DOI: 10.1039/c7bm00251c [DOI] [PubMed] [Google Scholar]
- 203.Shi L, Xue J, Zhao W, Wei X, Zhang M, Li L, et al. The prognosis of diabetic foot ulcer is independent of age? a comparative analysis of the characteristics of patients with diabetic foot ulcer in different age groups: A cross-sectional study from China. Int J Low Extrem Wounds. 2022:15347346221125844. DOI: 10.1177/15347346221125844 [DOI] [PubMed]
- 204.Silva JC, Pitta MGR, Pitta IR, Koh TJ, Abdalla DSP. New peroxisome proliferator-activated receptor agonist (GQ-11) improves wound healing in diabetic mice. Adv Wound Care (New Rochelle). 2019;8(9):417–28. DOI: 10.1089/wound.2018.0911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Spreadborough PJ, Strong AL, Mares J, Levi B, Davis TA. Tourniquet use following blast-associated complex lower limb injury and traumatic amputation promotes end organ dysfunction and amplified heterotopic ossification formation. J Orthop Surg Res. 2022;17(1):422. DOI: 10.1186/s13018-022-03321-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Squiers JJ, Thatcher JE, Bastawros DS, Applewhite AJ, Baxter RD, Yi F, et al. Machine learning analysis of multispectral imaging and clinical risk factors to predict amputation wound healing. J Vasc Surg. 2022;75(1):279–85. DOI: 10.1016/j.jvs.2021.06.478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Strong AL, Spreadborough PJ, Dey D, Yang P, Li S, Lee A, et al. BMP Ligand Trap ALK3-Fc Attenuates osteogenesis and heterotopic ossification in blast-related lower extremity trauma. Stem Cells Dev. 2021;30(2):91–105. DOI: 10.1089/scd.2020.0162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Sun X, Wang X, Zhao Z, Chen J, Li C, Zhao G. Paeoniflorin accelerates foot wound healing in diabetic rats though activating the Nrf2 pathway. Acta Histochemica. 2020;122(8):151649. DOI: 10.1016/j.acthis.2020.151649 [DOI] [PubMed] [Google Scholar]
- 209.Tan SS, Yeo XY, Liang ZC, Sethi SK, Tay SSW. Stromal vascular fraction promotes fibroblast migration and cellular viability in a hyperglycemic microenvironment through up-regulation of wound healing cytokines. Exp Mol Pathol. 2018;104(3):250–5. DOI: 10.1016/j.yexmp.2018.03.007 [DOI] [PubMed] [Google Scholar]
- 210.Tan WS, Arulselvan P, Ng SF, Mat Taib CN, Sarian MN, Fakurazi S. Improvement of diabetic wound healing by topical application of Vicenin-2 hydrocolloid film on Sprague Dawley rats. BMC Complement Altern Med. 2019;19(1):20. DOI: 10.1186/s12906-018-2427-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Tanaka K, Tanaka S, Okazaki J, Mii S. Preoperative nutritional status is independently associated with wound healing in patients undergoing open surgery for ischemic tissue loss. Vascular. 2021;29(6):897–904. DOI: 10.1177/1708538120980216 [DOI] [PubMed] [Google Scholar]
- 212.Vangaveti VN, Jhamb S, Hayes O, Goodall J, Bulbrook J, Robertson K, et al. Effects of vildagliptin on wound healing and markers of inflammation in patients with type 2 diabetic foot ulcer: a prospective, randomized, double-blind, placebo-controlled, single-center study. Diabetol Metab Syndr. 2022;14(1):183. DOI: 10.1186/s13098-022-00938-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Vatankhah N, Jahangiri Y, Landry GJ, McLafferty RB, Alkayed NJ, Moneta GL, et al. Predictive value of neutrophil-to-lymphocyte ratio in diabetic wound healing. J Vasc Surg. 2017;65(2):478–83. DOI: 10.1016/j.jvs.2016.08.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Vieceli Dalla Sega F, Cimaglia P, Manfrini M, Fortini F, Marracino L, Bernucci D, et al. Circulating biomarkers of endothelial dysfunction and inflammation in predicting clinical outcomes in diabetic patients with critical limb ischemia. Int J Mol Sci. 2022;23(18). DOI: 10.3390/ijms231810641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wang H, Wang X, Liu X, Zhou J, Yang Q, Chai B, et al. miR-199a-5p Plays a pivotal role on wound healing via suppressing vegfa and rock1 in diabetic ulcer foot. Oxid Med Cell Longev. 2022;2022:4791059. DOI: 10.1155/2022/4791059 [DOI] [PMC free article] [PubMed]
- 216.Woo Y, Suh YJ, Lee H, Jeong E, Park SC, Yun SS, et al. TcPO2 value can predict wound healing time in clinical practice of CLTI patients. Ann Vasc Surg. 2023;91:249–56. DOI: 10.1016/j.avsg.2022.11.020 [DOI] [PubMed] [Google Scholar]
- 217.Wu M, Yu Z, Matar DY, Karvar M, Chen Z, Ng B, et al. Human amniotic membrane promotes angiogenesis in an oxidative stress chronic diabetic murine wound model. Adv Wound Care (New Rochelle). 2023;12(6):301–15. DOI: 10.1089/wound.2022.0005 [DOI] [PubMed] [Google Scholar]
- 218.Xiang X, Chen J, Jiang T, Yan C, Kang Y, Zhang M, et al. Milk-derived exosomes carrying siRNA-KEAP1 promote diabetic wound healing by improving oxidative stress. Drug Deliv Transl Res. 2023;13(9):2286–96. DOI: 10.1007/s13346-023-01306-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Yan J, Tie G, Wang S, Tutto A, DeMarco N, Khair L, et al. Diabetes impairs wound healing by Dnmt1-dependent dysregulation of hematopoietic stem cells differentiation towards macrophages. Nat Commun. 2018;9(1):33. DOI: 10.1038/s41467-017-02425-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Yang S, Gu Z, Lu C, Zhang T, Guo X, Xue G, et al. Neutrophil extracellular traps are markers of wound healing impairment in patients with diabetic foot ulcers treated in a multidisciplinary setting. Adv Wound Care (New Rochelle). 2020;9(1):16–27. DOI: 10.1089/wound.2019.0943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Yang X, Mathis BJ, Huang Y, Li W, Shi Y. KLF4 Promotes diabetic chronic wound healing by suppressing Th17 cell differentiation in an MDSC-dependent manner. J Diabetes Res. 2021;2021:7945117. DOI: 10.1155/2021/7945117 [DOI] [PMC free article] [PubMed]
- 222.Yang Y, Hu H, Wang W, Duan X, Luo S, Wang X, et al. The identification of functional proteins from amputated lumbricus Eisenia fetida on the wound healing process. Biomed Pharmacother. 2017;95:1469–78. DOI: 10.1016/j.biopha.2017.09.049 [DOI] [PubMed] [Google Scholar]
- 223.Zhang F, Liu Y, Wang S, Yan X, Lin Y, Chen D, et al. Interleukin-25-mediated-IL-17RB upregulation promotes cutaneous wound healing in diabetic mice by improving endothelial cell functions. Front Immunol. 2022;13:809755. DOI: 10.3389/fimmu.2022.809755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Zhang S, Wang S, Xu L, He Y, Xiang J, Tang Z. Clinical outcomes of transmetatarsal amputation in patients with diabetic foot ulcers treated without revascularization. Diabetes Ther. 2019;10(4):1465–72. DOI: 10.1007/s13300-019-0653-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zhang Y, Jiang W, Kong L, Fu J, Zhang Q, Liu H. PLGA@IL-8 nanoparticles-loaded acellular dermal matrix as a delivery system for exogenous MSCs in diabetic wound healing. Int J Biol Macromol. 2023;224:688–98. DOI: 10.1016/j.ijbiomac.2022.10.157 [DOI] [PubMed] [Google Scholar]
- 226.Zhao Y, Luo L, Huang L, Zhang Y, Tong M, Pan H, et al. In situ hydrogel capturing nitric oxide microbubbles accelerates the healing of diabetic foot. J Control Release. 2022;350:93–106. DOI: 10.1016/j.jconrel.2022.08.018 [DOI] [PubMed] [Google Scholar]
- 227.Zhao Y, Wang X, Yang S, Song X, Sun N, Chen C, et al. Kanglexin accelerates diabetic wound healing by promoting angiogenesis via FGFR1/ERK signaling. Biomed Pharmacother. 2020;132:110933. DOI: 10.1016/j.biopha.2020.110933 [DOI] [PubMed] [Google Scholar]
- 228.Zheng Z, Liu Y, Yang Y, Tang J, Cheng B. Topical 1% propranolol cream promotes cutaneous wound healing in spontaneously diabetic mice. Wound Repair Regen. 2017;25(3):389–97. DOI: 10.1111/wrr.12546 [DOI] [PubMed] [Google Scholar]
- 229.Zhu Z, Wang L, Peng Y, Xiaoying C, Zhou L, Jin Y, et al. Continuous self-oxygenated double-layered hydrogel under natural light for real-time infection monitoring, enhanced photodynamic therapy, and hypoxia relief in refractory diabetic wounds healing. Adv Funct Mater. 2022;32. DOI: 10.1002/adfm.202201875 [DOI]
- 230.Msskkhksmiazk Dur-E-Sameen. Exploring association of anemia with diabetic foot ulcer and its impact on disease outcome in a tertiary care hospital. Pak J Med Health Sci. 2023;16(12):406. DOI: 10.53350/pjmhs20221612406 [DOI] [Google Scholar]
- 231.Salih K, Faraj YF, Mohammed I. Salivary matrix metalloproteinase-8 indicate the severity of diabetic foot ulcer. Int J Biochem Cell Biol. 2020;18:1543–8. [Google Scholar]
- 232.Kevin L, Jagadeesh M, Priscilla L, Kacie K, Richard S, Edwin R, et al. Oxygenation based perfusion assessment of diabetic foot ulcers using a breath-hold paradigm. ProcSPIE. 2019;10873:1087304. DOI: 10.1117/12.2509917 [DOI] [Google Scholar]
- 233.Ramaprabha P, Ramani CP, Kesavan R. Study on microbiome of chronic non healing diabetic ulcers with special reference to biofilm and multidrug resistant strains. J Clin Diagn Res. 2021. DOI: 10.7860/JCDR/2021/50126.15471 [DOI]
- 234.Strauss C, Anker A, Klein S, Kemper R, Brebant V, Prantl L, et al. Monitoring free flaps and replanted digits via perfusion index - A proof of concept study. Clin Hemorheol Microcirc. 2022;80(4):363–71. DOI: 10.3233/ch-211295 [DOI] [PubMed] [Google Scholar]
- 235.Van Den Hoven P, Van Den Berg SD, Van Der Valk JP, Van Der Krogt H, Van Doorn LP, Van De Bogt KEA, et al. Assessment of tissue viability following amputation surgery using near-infrared fluorescence imaging with indocyanine green. Ann Vasc Surg. 2022;78:281–7. DOI: 10.1016/j.avsg.2021.04.030 [DOI] [PubMed] [Google Scholar]
- 236.Zubair M, Ahmad J. Transcutaneous oxygen pressure (TcPO(2)) and ulcer outcome in diabetic patients: Is there any correlation? Diabetes Metab Syndr. 2019;13(2):953–8. DOI: 10.1016/j.dsx.2018.12.008 [DOI] [PubMed] [Google Scholar]
- 237.Chen CY, Wu RW, Hsu MC, Hsieh CJ, Chou MC. Adjunctive hyperbaric oxygen therapy for healing of chronic diabetic foot ulcers: A randomized controlled trial. J Wound Ostomy Continence Nurs. 2017;44(6):536–45. DOI: 10.1097/won.0000000000000374 [DOI] [PubMed] [Google Scholar]
- 238.Izadi M, Kheirjou R, Mohammadpour R, Aliyoldashi MH, Moghadam SJ, Khorvash F, et al. Efficacy of comprehensive ozone therapy in diabetic foot ulcer healing. Diabetes Metab Syndr. 2019;13(1):822–5. DOI: 10.1016/j.dsx.2018.11.060 [DOI] [PubMed] [Google Scholar]
- 239.Oley MH, Oley MC, Noersasongko AD, Hatta M, Philips GG, Agustine, et al. Effects of hyperbaric oxygen therapy on vascular endothelial growth factor protein and mRNA in crush injury patients: A randomized controlled trial study. Int J Surg Open. 2021;29:33–9. DOI: 10.1016/j.ijso.2021.01.003 [DOI] [Google Scholar]
- 240.Oley MH, Oley MC, Tjandra DE, Sedu SW, Sumarauw ERN, Aling DMR, et al. Hyperbaric oxygen therapy in the healing process of foot ulcers in diabetic type 2 patients marked by interleukin 6, vascular endothelial growth factor, and PEDIS score: A randomized controlled trial study. Int J Surg Open. 2020;27:154–61. DOI: 10.1016/j.ijso.2020.11.012 [DOI] [Google Scholar]
- 241.Viswanathan V, Juttada U, Babu M. Efficacy of recombinant human epidermal growth factor (Regen-D 150) in healing diabetic foot ulcers: A hospital-based randomized controlled trial. Int J Low Extrem Wounds. 2020;19(2):158–64. DOI: 10.1177/1534734619892791 [DOI] [PubMed] [Google Scholar]
- 242.Chiang N, Rodda OA, Sleigh J, Vasudevan T. Effects of topical negative pressure therapy on tissue oxygenation and wound healing in vascular foot wounds. J Vasc Surg. 2017;66(2):564–71. DOI: 10.1016/j.jvs.2017.02.050 [DOI] [PubMed] [Google Scholar]
- 243.Nolan GS, Smith OJ, Heavey S, Jell G, Mosahebi A. Histological analysis of fat grafting with platelet-rich plasma for diabetic foot ulcers-A randomised controlled trial. Int Wound J. 2022;19(2):389–98. DOI: 10.1111/iwj.13640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Nur Rosyid F, Dharmana E, Suwondo A, Hs K, Sugiarto S. The effect of bitter melon (Momordica Charantia L.) leaves extract on TNF-α serum levels and diabetic foot ulcers improvement: Randomized controlled trial. Biomed Pharmacol J. 2018;11:1413–21. DOI: 10.13005/bpj/1505 [DOI] [Google Scholar]
- 245.Chen J, Bao X, Meng T, Sun J, Yang X. Zeolitic imidazolate framework-67 accelerates infected diabetic chronic wound healing. Chem Eng J. 2022;430:133091. DOI: 10.1016/j.cej.2021.133091 [DOI] [Google Scholar]
- 246.Chen Z, Haus JM, DiPietro LA, Koh TJ, Minshall RD. Neutralization of excessive CCL28 improves wound healing in diabetic mice. Front Pharmacol. 2023;14:1087924. DOI: 10.3389/fphar.2023.1087924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Derakhshandeh H, Aghabaglou F, McCarthy A, Mostafavi A, Wiseman C, Bonick Z, et al. A wirelessly controlled smart bandage with 3d-printed miniaturized needle arrays. Adv Funct Mater. 2020;30(13). DOI: 10.1002/adfm.201905544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Gao S, Chen T, Wang Z, Ji P, Xu L, Cui W, et al. Immuno-activated mesenchymal stem cell living electrospun nanofibers for promoting diabetic wound repair. J Nanobiotechnology. 2022;20(1):294. DOI: 10.1186/s12951-022-01503-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Greene CJ, Anderson S, Barthels D, Howlader MSI, Kanji S, Sarkar J, et al. DPSC products accelerate wound healing in diabetic mice through induction of SMAD molecules. Cells. 2022;11(15). DOI: 10.3390/cells11152409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Hassan RF, Kadhim HM. Comparative effects of phenolic extract as an ointment dosage form in inducing wound healing in mice and β-sitosterol in experimentally induced acute wound healing in mice. J Pharm Negat Results. 2022;13(3):194–203. DOI: 10.47750/pnr.2022.13.03.031 [DOI] [Google Scholar]
- 251.Jing S, Li H, Xu H. Mesenchymal stem cell derived exosomes therapy in diabetic wound repair. Int J Nanomedicine. 2023;18:2707–20. DOI: 10.2147/ijn.S411562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Kanji S, Das M, Joseph M, Aggarwal R, Sharma SM, Ostrowski M, et al. Nanofiber-expanded human CD34(+) cells heal cutaneous wounds in streptozotocin-induced diabetic mice. Sci Rep. 2019;9(1):8415. DOI: 10.1038/s41598-019-44932-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Khan MS, Tauqeer Ahmed M. Novel candidates for chronic diabetic wound healing. J Pak Assoc Dermatol. 2022;32(3):526–31. [Google Scholar]
- 254.Li X, Xie X, Lian W, Shi R, Han S, Zhang H, et al. Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp Mol Med. 2018;50(4):1–14. DOI: 10.1038/s12276-018-0058-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Liu C, Teo MHY, Pek SLT, Wu X, Leong ML, Tay HM, et al. A multifunctional role of leucine-rich α-2-glycoprotein 1 in cutaneous wound healing under normal and diabetic conditions. Diabetes. 2020;69(11):2467–80. DOI: 10.2337/db20-0585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Paul TS, Das BB, Talekar YP, Banerjee S. Exploration of the role of a lithophytic fern, Pteris vittata L. in wound tissue regeneration and remodelling of genes in hyperglycaemic rat model. Clinical Phytoscience. 2020;6(1):79. DOI: 10.1186/s40816-020-00223-7 [DOI] [Google Scholar]
- 257.Tellechea A, Bai S, Dangwal S, Theocharidis G, Nagai M, Koerner S, et al. Topical application of a mast cell stabilizer improves impaired diabetic wound healing. J Invest Dermatol. 2020;140(4):901–11.e11. DOI: 10.1016/j.jid.2019.08.449 [DOI] [PubMed] [Google Scholar]
- 258.Tkaczyk C, Jones-Nelson O, Shi YY, Tabor DE, Cheng L, Zhang T, et al. Neutralizing staphylococcus aureus virulence with AZD6389, a three mab combination, accelerates closure of a diabetic polymicrobial wound. mSphere. 2022;7(3):e0013022. DOI: 10.1128/msphere.00130-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Wang T, Zheng Y, Shi Y, Zhao L. pH-responsive calcium alginate hydrogel laden with protamine nanoparticles and hyaluronan oligosaccharide promotes diabetic wound healing by enhancing angiogenesis and antibacterial activity. Drug Deliv Transl Res. 2019;9(1):227–39. DOI: 10.1007/s13346-018-00609-8 [DOI] [PubMed] [Google Scholar]
- 260.Xia G, Liu Y, Tian M, Gao P, Bao Z, Bai X, et al. Nanoparticles/thermosensitive hydrogel reinforced with chitin whiskers as a wound dressing for treating chronic wounds. J Mater Chem B. 2017;5(17):3172–85. DOI: 10.1039/c7tb00479f [DOI] [PubMed] [Google Scholar]
- 261.Yadav S, Arya DK, Pandey P, Anand S, Gautam AK, Ranjan S, et al. ECM mimicking biodegradable nanofibrous scaffold enriched with Curcumin/ZnO to accelerate diabetic wound healing via multifunctional bioactivity. Int J Nanomedicine. 2022;17:6843–59. DOI: 10.2147/ijn.S388264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Ye J, Kang Y, Sun X, Ni P, Wu M, Lu S. MicroRNA-155 inhibition promoted wound healing in diabetic rats. Int J Low Extrem Wounds. 2017;16(2):74–84. DOI: 10.1177/1534734617706636 [DOI] [PubMed] [Google Scholar]
- 263.Zahid AA, Ahmed R, Ur Rehman SR, Augustine R, Hasan A. Reactive nitrogen species releasing hydrogel for enhanced wound healing. Annu Int Conf IEEE Eng Med Biol Soc. 2019;2019:3939–42. DOI: 10.1109/embc.2019.8856469 [DOI] [PubMed]
- 264.Di Cristo F, Valentino A, De Luca I, Peluso G, Bonadies I, Di Salle A, et al. Polylactic acid/poly(vinylpyrrolidone) co-electrospun fibrous membrane as a tunable quercetin delivery platform for diabetic wounds. Pharmaceutics. 2023;15(3). DOI: 10.3390/pharmaceutics15030805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Kim JH, Ruegger PR, Lebig EG, VanSchalkwyk S, Jeske DR, Hsiao A, et al. High levels of oxidative stress create a microenvironment that significantly decreases the diversity of the microbiota in diabetic chronic wounds and promotes biofilm formation. Front Cell Infect Microbiol. 2020;10:259. DOI: 10.3389/fcimb.2020.00259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Nguyen TT, Jones JI, Wolter WR, Pérez RL, Schroeder VA, Champion MM, et al. Hyperbaric oxygen therapy accelerates wound healing in diabetic mice by decreasing active matrix metalloproteinase-9. Wound Repair Regen. 2020;28(2):194–201. DOI: 10.1111/wrr.12782 [DOI] [PubMed] [Google Scholar]
- 267.Shang W, Chen G, Li Y, Zhuo Y, Wang Y, Fang Z, et al. Static magnetic field accelerates diabetic wound healing by facilitating resolution of inflammation. J Diabetes Res. 2019;2019:5641271. DOI: 10.1155/2019/5641271 [DOI] [PMC free article] [PubMed]
- 268.Ojalvo AG, Acosta JB, Marí YM, Mayola MF, Pérez CV, Gutiérrez WS, et al. Healing enhancement of diabetic wounds by locally infiltrated epidermal growth factor is associated with systemic oxidative stress reduction. Int Wound J. 2017;14(1):214–25. DOI: 10.1111/iwj.12592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Al-Shibly I, Alhamdany M, Al-Kaif R, Al-Kaif L. Immunological base behind the increased susceptibility of diabetic patients for infections. Indian J Public Health Res Dev. 2019;10:3047. DOI: 10.5958/0976-5506.2019.03343.6 [DOI] [Google Scholar]
- 270.Camacho-Rodríguez H, Guillen-Pérez IA, Roca-Campaña J, Baldomero-Hernández JE, Tuero-Iglesias Á D, Galván-Cabrera JA, et al. Heberprot-P's effect on gene expression in healing diabetic foot ulcers. Medicc Rev. 2018;20(3):10–4. DOI: 10.37757/mr2018.V20.N3.4 [DOI] [PubMed] [Google Scholar]
- 271.García-Ojalvo A, Berlanga Acosta J, Figueroa-Martínez A, Béquet-Romero M, Mendoza-Marí Y, Fernández-Mayola M, et al. Systemic translation of locally infiltrated epidermal growth factor in diabetic lower extremity wounds. Int Wound J. 2019;16(6):1294–303. DOI: 10.1111/iwj.13189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Naderi N, Zaefizadeh M. Expression of growth factors in re-epithelialization of diabetic foot ulcers after treatment with non-thermal plasma radiation. Biomedical Research (India). 2017;28:3402–7. [Google Scholar]
- 273.Pu D, Lei X, Leng W, Zheng Y, Chen L, Liang Z, et al. Lower limb arterial intervention or autologous platelet-rich gel treatment of diabetic lower extremity arterial disease patients with foot ulcers. Ann Transl Med. 2019;7(18):485. DOI: 10.21037/atm.2019.07.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Baumfeld D, Baumfeld T, Macedo B, Zambelli R, Lopes F, Nery C. Factors related to amputation level and wound healing in diabetic patients. Acta Ortop Bras. 2018;26(5):342–5. DOI: 10.1590/1413-785220182605173445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Kapukaya R, Kapukaya A, Keklikcioglu B, Özdemir AA. How important are mean platelet volume and neutrophil values in diabetic foot amputation decision? Eur J Plast Surg. 2021;44(4):507–10. DOI: 10.1007/s00238-020-01773-2 [DOI] [Google Scholar]
- 276.Morisaki K, Yamaoka T, Iwasa K. Risk factors for wound complications and 30-day mortality after major lower limb amputations in patients with peripheral arterial disease. Vascular. 2018;26(1):12–7. DOI: 10.1177/1708538117714197 [DOI] [PubMed] [Google Scholar]
- 277.Trejo J, Ryan E, Khan F, Iannuzzi N, Chansky H, Lack WD. Risk factors for failure of limb salvage among veterans with foot ulcers. Foot Ankle Surg. 2022;28(5):584–7. DOI: 10.1016/j.fas.2021.06.003 [DOI] [PubMed] [Google Scholar]
- 278.Wu T, Xie D, Zhao X, Xu M, Luo L, Deng D, et al. Enhanced expression of miR-34c in peripheral plasma associated with diabetic foot ulcer in type 2 diabetes patients. Diabetes Metab Syndr Obes. 2021;14:4263–73. DOI: 10.2147/dmso.S326066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Yang AE, Hartranft CA, Reiss A, Holden CR. Improving outcomes for lower extremity amputations using intraoperative fluorescent angiography to predict flap viability. Vasc Endovascular Surg. 2018;52(1):16–21. DOI: 10.1177/1538574417740048 [DOI] [PubMed] [Google Scholar]
- 280.JBI levels of evidence [Internet]. Joanna Briggs Institute. 2013; [cited 2024, July 5]. Available from: https://jbi.global/sites/default/files/2019-05/JBI-Levels-of-evidence_2014_0.pdf [Google Scholar]
- 281.Metcalf D, Haalboom M, Bowler P, Gamerith C, Sigl E, Hasmann Heinzle A, et al. Elevated wound fluid pH correlates with increased risk of wound infection. Wound Medicine. 2019;26:100166. DOI: 10.1016/j.wndm.2019.100166 [DOI] [Google Scholar]
- 282.Shah P, Inturi R, Anne D, Jadhav D, Viswambharan V, Khadilkar R, et al. Wagner's classification as a tool for treating diabetic foot ulcers: Our observations at a suburban teaching hospital. Cureus. 2022;14(1):e21501. DOI: 10.7759/cureus.21501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.How many studies should be included in a systematic review? [internet]. DistillerSR Inc. [cited 2024, July 5]. Available from: https://www.distillersr.com/resources/systematic-literature-reviews/how-many-studies-should-be-included-in-a-systematic-review [Google Scholar]
- 284.Development WOfEC-oa. Low- and middle-income countries Internet: Wellcome; 2024. [Available from: https://wellcome.org/grant-funding/guidance/prepare-to-apply/low-and-middle-income-countries
- 285.Yao B. International research collaboration: Challenges and opportunities. J Diagn Med Sonogr. 2021;37(2):107–8. DOI: 10.1177/8756479320976130 [DOI] [Google Scholar]
- 286.Franzen SR, Chandler C, Lang T. Health research capacity development in low and middle income countries: Reality or rhetoric? A systematic meta-narrative review of the qualitative literature. BMJ Open. 2017;7(1):e012332. DOI: 10.1136/bmjopen-2016-012332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Sen CK. Human wounds and its burden: An updated compendium of estimates. Adv Wound Care (New Rochelle). 2019;8(2):39–48. DOI: 10.1089/wound.2019.0946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Guest JF, Fuller GW, Vowden P. Cohort study evaluating the burden of wounds to the UK's National Health Service in 2017/2018: update from 2012/2013. BMJ Open. 2020;10(12):e045253. DOI: 10.1136/bmjopen-2020-045253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Tricco AC, Lillie E, Zarin W, O'Brien K, Colquhoun H, Kastner M, et al. A scoping review on the conduct and reporting of scoping reviews. BMC Med Res Methodol. 2016;16:15. DOI: 10.1186/s12874-016-0116-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Tod D, Booth A, Smith B. Critical appraisal. Int Rev Sport Exerc Psychol. 2022;15(1):52–72. DOI: 10.1080/1750984X.2021.1952471 [DOI] [Google Scholar]
- 291.Speich B, von Niederhäusern B, Schur N, Hemkens LG, Fürst T, Bhatnagar N, et al. Systematic review on costs and resource use of randomized clinical trials shows a lack of transparent and comprehensive data. J Clin Epidemiol. 2018;96:1–11. DOI: 10.1016/j.jclinepi.2017.12.018 [DOI] [PubMed] [Google Scholar]
- 292.Morris ZS, Wooding S, Grant J. The answer is 17 years, what is the question: Understanding time lags in translational research. J R Soc Med. 2011;104(12):510–20. DOI: 10.1258/jrsm.2011.110180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Wound management clinical practice guidelines tissue viability service [Internet]. East London. 2019; [cited 2024, July 5]. Available from: https://www.elft.nhs.uk/sites/default/files/Wound%20Management%20Guidelines%206.0.pdf [Google Scholar]
- 294.Bryant JL, Brooks TL, Schmidt B, Mostow EN. Reliability of wound measuring techniques in an outpatient wound center. Ostomy Wound Manage. 2001;47(4):44–51. [PubMed] [Google Scholar]
- 295.Keast DH, Bowering CK, Evans AW, Mackean GL, Burrows C, D'Souza L. Measure: A proposed assessment framework for developing best practice recommendations for wound assessment. Wound Repair Regen. 2004;12(3 Suppl):S1–17. DOI: 10.1111/j.1067-1927.2004.0123S1.x [DOI] [PubMed] [Google Scholar]
- 296.Shah A, Wollak C, Shah JB. Wound measurement techniques: Comparing the use of ruler method, 2D imaging and 3D scanner. J Am Coll Clin Wound Spec. 2013;5(3):52–7. DOI: 10.1016/j.jccw.2015.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Khalid KA, Nawi AFM, Zulkifli N, Barkat MA, Hadi H. Aging and wound healing of the skin: A review of clinical and pathophysiological hallmarks. Life (Basel). 2022;12(12). DOI: 10.3390/life12122142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Engeland CG, Sabzehei B, Marucha PT. Sex hormones and mucosal wound healing. Brain Behav Immun. 2009;23(5):629–35. DOI: 10.1016/j.bbi.2008.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Jung MK, Callaci JJ, Lauing KL, Otis JS, Radek KA, Jones MK, et al. Alcohol exposure and mechanisms of tissue injury and repair. Alcohol Clin Exp Res. 2011;35(3):392–9. DOI: 10.1111/j.1530-0277.2010.01356.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.McDaniel JC, Browning KK. Smoking, chronic wound healing, and implications for evidence-based practice. J Wound Ostomy Continence Nurs. 2014;41(5):415–23; quiz E1-2. DOI: 10.1097/won.0000000000000057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Stechmiller JK. Understanding the role of nutrition and wound healing. Nutr Clin Pract. 2010;25(1):61–8. DOI: 10.1177/0884533609358997 [DOI] [PubMed] [Google Scholar]
- 302.Robson MC. Wound infection. A failure of wound healing caused by an imbalance of bacteria. Surg Clin North Am. 1997;77(3):637–50. DOI: 10.1016/s0039-6109(05)70572-7 [DOI] [PubMed] [Google Scholar]
- 303.Rodriguez PG, Felix FN, Woodley DT, Shim EK. The role of oxygen in wound healing: A review of the literature. Dermatol Surg. 2008;34(9):1159–69. DOI: 10.1111/j.1524-4725.2008.34254.x [DOI] [PubMed] [Google Scholar]
- 304.Raffetto JD, Ligi D, Maniscalco R, Khalil RA, Mannello F. Why venous leg ulcers have difficulty healing: Overview on pathophysiology, clinical consequences, and treatment. J Clin Med. 2020;10(1). DOI: 10.3390/jcm10010029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Yepson H, Mazzone B, Eskridge S, Shannon K, Awodele E, Farrokhi S, et al. The influence of tobacco use, alcohol consumption, and weight gain on development of secondary musculoskeletal injury after lower limb amputation. Arch Phys Med Rehabil. 2020;101(10):1704–10. DOI: 10.1016/j.apmr.2020.04.022 [DOI] [PubMed] [Google Scholar]
- 306.Lind J, Kramhøft M, Bødtker S. The influence of smoking on complications after primary amputations of the lower extremity. Clin Orthop Relat Res. 1991(267):211–7. [PubMed] [Google Scholar]
- 307.Nishio Y, Tsuji Y, Kitano I, Terashi H. Influence of peripheral arterial disease on wound healing in heel pressure ulcers. Kobe J Med Sci. 2022;67(4):E146–e54. [PMC free article] [PubMed] [Google Scholar]
- 308.Churilov I, Churilov L, Murphy D. Do rigid dressings reduce the time from amputation to prosthetic fitting? A systematic review and meta-analysis. Ann Vasc Surg. 2014;28(7):1801–8. DOI: 10.1016/j.avsg.2014.05.002 [DOI] [PubMed] [Google Scholar]
- 309.Mathew-Steiner SS, Roy S, Sen CK. Collagen in wound healing. Bioengineering (Basel). 2021;8(5). DOI: 10.3390/bioengineering8050063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Cattano NM, Driban JB, Cameron KL, Sitler MR. Impact of physical activity and mechanical loading on biomarkers typically used in osteoarthritis assessment: current concepts and knowledge gaps. Ther Adv Musculoskelet Dis. 2017;9(1):11–21. DOI: 10.1177/1759720x16670613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Boyko EJ, Zelnick LR, Braffett BH, Pop-Busui R, Cowie CC, Lorenzi GM, et al. Risk of foot ulcer and lower-extremity amputation among participants in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care. 2022;45(2):357–64. DOI: 10.2337/dc21-1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.De Silva GS, Saffaf K, Sanchez LA, Zayed MA. Amputation stump perfusion is predictive of post-operative necrotic eschar formation. Am J Surg. 2018;216(3):540–6. DOI: 10.1016/j.amjsurg.2018.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Yin V, Cobb JP, Wightman SC, Atay SM, Harano T, Kim AW. Centers for disease control (CDC) wound classification is prognostic of 30-day readmission following surgery. World J Surg. 2023;47(10):2392–400. DOI: 10.1007/s00268-023-07093-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Do HTT, Edwards H, Finlayson K. Surgical wound assessment tool: Construct validity and inter-rater reliability of a tool designed for nurses. J Clin Nurs. 2023;32(1-2):83–95. DOI: 10.1111/jocn.16476 [DOI] [PubMed] [Google Scholar]
- 315.Firth K, Smith K, Sakallaris BR, Bellanti DM, Crawford C, Avant KC. Healing, a concept analysis. Glob Adv Health Med. 2015;4(6):44–50. DOI: 10.7453/gahmj.2015.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Doering LV, Moser DK, Lemankiewicz W, Luper C, Khan S. Depression, healing, and recovery from coronary artery bypass surgery. Am J Crit Care. 2005;14(4):316–24. [PubMed] [Google Scholar]
- 317.Sahu A, Sagar R, Sarkar S, Sagar S. Psychological effects of amputation: A review of studies from India. Ind Psychiatry J. 2016;25(1):4–10. DOI: 10.4103/0972-6748.196041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Peters CML, de Vries J, Steunenberg SL, Ho GH, Lodder P, van der Laan L. Is There an important role for anxiety and depression in the elderly patients with critical limb ischemia, especially after major amputation? Ann Vasc Surg. 2019;58:142–50. DOI: 10.1016/j.avsg.2018.10.045 [DOI] [PubMed] [Google Scholar]
- 319.McElligott D. Healing: the journey from concept to nursing practice. J Holist Nurs. 2010;28(4):251–9. DOI: 10.1177/0898010110376321 [DOI] [PubMed] [Google Scholar]
- 320.Levin J. What is “Healing”?: Reflections on diagnostic criteria, nosology, and etiology. Explore (NY). 2017;13(4):244–56. DOI: 10.1016/j.explore.2017.04.005 [DOI] [PubMed] [Google Scholar]
- 321.Smith DG, McFarland LV, Sangeorzan BJ, Reiber GE, Czerniecki JM. Postoperative dressing and management strategies for transtibial amputations: A critical review. J Rehabil Res Dev. 2003;40(3):213–24. [PubMed] [Google Scholar]
- 322.Song Y, Jo Y, Sohn J, Kim R. A pilot study to explore a correlation between inflammatory markers and the wound healing rate in diabetic patients. Medicina (Kaunas). 2022;58(3). DOI: 10.3390/medicina58030390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Arsenault KA, Al-Otaibi A, Devereaux PJ, Thorlund K, Tittley JG, Whitlock RP. The use of transcutaneous oximetry to predict healing complications of lower limb amputations: A systematic review and meta-analysis. Eur J Vasc Endovasc Surg. 2012;43(3):329–36. DOI: 10.1016/j.ejvs.2011.12.004 [DOI] [PubMed] [Google Scholar]
- 324.Matsumoto M, Nakagami G, Kitamura A, Kurita M, Suga H, Miyake T, et al. Ultrasound assessment of deep tissue on the wound bed and periwound skin: A classification system using ultrasound images. J Tissue Viability. 2021;30(1):28–35. DOI: 10.1016/j.jtv.2020.08.002 [DOI] [PubMed] [Google Scholar]
- 325.Patel S, Ershad F, Zhao M, Isseroff RR, Duan B, Zhou Y, et al. Wearable electronics for skin wound monitoring and healing. Soft Sci. 2022;2. DOI: 10.20517/ss.2022.13 [DOI] [PMC free article] [PubMed]
- 326.Hawamdeh ZM, Othman YS, Ibrahim AI. Assessment of anxiety and depression after lower limb amputation in Jordanian patients. Neuropsychiatr Dis Treat. 2008;4(3):627–33. DOI: 10.2147/ndt.s2541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Singh R, Ripley D, Pentland B, Todd I, Hunter J, Hutton L, et al. Depression and anxiety symptoms after lower limb amputation: the rise and fall. Clin Rehabil. 2009;23(3):281–6. DOI: 10.1177/0269215508094710 [DOI] [PubMed] [Google Scholar]
- 328.Nikolajsen L, Ilkjaer S, Krøner K, Christensen JH, Jensen TS. The influence of preamputation pain on postamputation stump and phantom pain. Pain. 1997;72(3):393–405. DOI: 10.1016/s0304-3959(97)00061-4 [DOI] [PubMed] [Google Scholar]
- 329.PLUS-M [Internet]. University of Washington. 2013; [cited 2024, July 5]. Available from: https://plus-m.org/ [Google Scholar]
- 330.Ambler GK, Brookes-Howell L, Jones JAR, Verma N, Bosanquet DC, Thomas-Jones E, et al. Development of core outcome sets for people undergoing major lower limb amputation for complications of peripheral vascular disease. Eur J Vasc Endovasc Surg. 2020;60(5):730–8. DOI: 10.1016/j.ejvs.2020.06.021 [DOI] [PubMed] [Google Scholar]
- 331.Gethin G, Ivory JD, Sezgin D, Muller H, O'Connor G, Vellinga A. What is the “normal” wound bed temperature? A scoping review and new hypothesis. Wound Repair Regen. 2021;29(5):843–7. DOI: 10.1111/wrr.12930 [DOI] [PubMed] [Google Scholar]
- 332.Derwin R, Patton D, Strapp H, Moore Z. Wound pH and temperature as predictors of healing: An observational study. J Wound Care. 2023;32(5):302–10. DOI: 10.12968/jowc.2023.32.5.302 [DOI] [PubMed] [Google Scholar]
- 333.Holzer-Geissler JCJ, Schwingenschuh S, Zacharias M, Einsiedler J, Kainz S, Reisenegger P, et al. The impact of prolonged inflammation on wound healing. Biomedicines. 2022;10(4). DOI: 10.3390/biomedicines10040856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Vaisson G, Provencher T, Dugas M, Trottier M, Chipenda Dansokho S, Colquhoun H, et al. User involvement in the design and development of patient decision aids and other personal health tools: A systematic review. Med Decis Making. 2021;41(3):261–74. DOI: 10.1177/0272989x20984134 [DOI] [PubMed] [Google Scholar]
- 335.Stoll CRT, Izadi S, Fowler S, Green P, Suls J, Colditz GA. The value of a second reviewer for study selection in systematic reviews. Res Synth Methods. 2019;10(4):539–45. DOI: 10.1002/jrsm.1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Suri, H. Ethical considerations of conducting systematic reviews in educational research. In: Zawacki-Richter, O., Kerres, M., Bedenlier, S., Bond, M., Buntins, K. (eds). systematic reviews in educational research. Springer VS, Wiesbaden. 2020. (41–54). DOI: 10.1007/978-3-658-27602-7_3 [DOI] [Google Scholar]
- 337.Van Nes F, Abma T, Jonsson H, Deeg D. Language differences in qualitative research: Is meaning lost in translation? Eur J Ageing. 2010;7(4):313–6. DOI: 10.1007/s10433-010-0168-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Paez A. Gray literature: An important resource in systematic reviews. J Evid Based Med. 2017;10(3):233–40. DOI: 10.1111/jebm.12266 [DOI] [PubMed] [Google Scholar]
- 339.Gazendam AM, Slawaska-Eng D, Nucci N, Bhatt O, Ghert M. The impact of industry funding on randomized controlled trials of biologic therapies. Medicines (Basel). 2022;9(3). DOI: 10.3390/medicines9030018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Lee L, Packer TL, Tang SH, Girdler S. Self-management education programs for age-related macular degeneration: A systematic review. Australas J Ageing. 2008;27(4):170–6. DOI: 10.1111/j.1741-6612.2008.00298.x [DOI] [PubMed] [Google Scholar]