Abstract
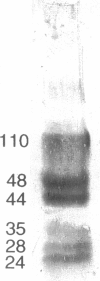
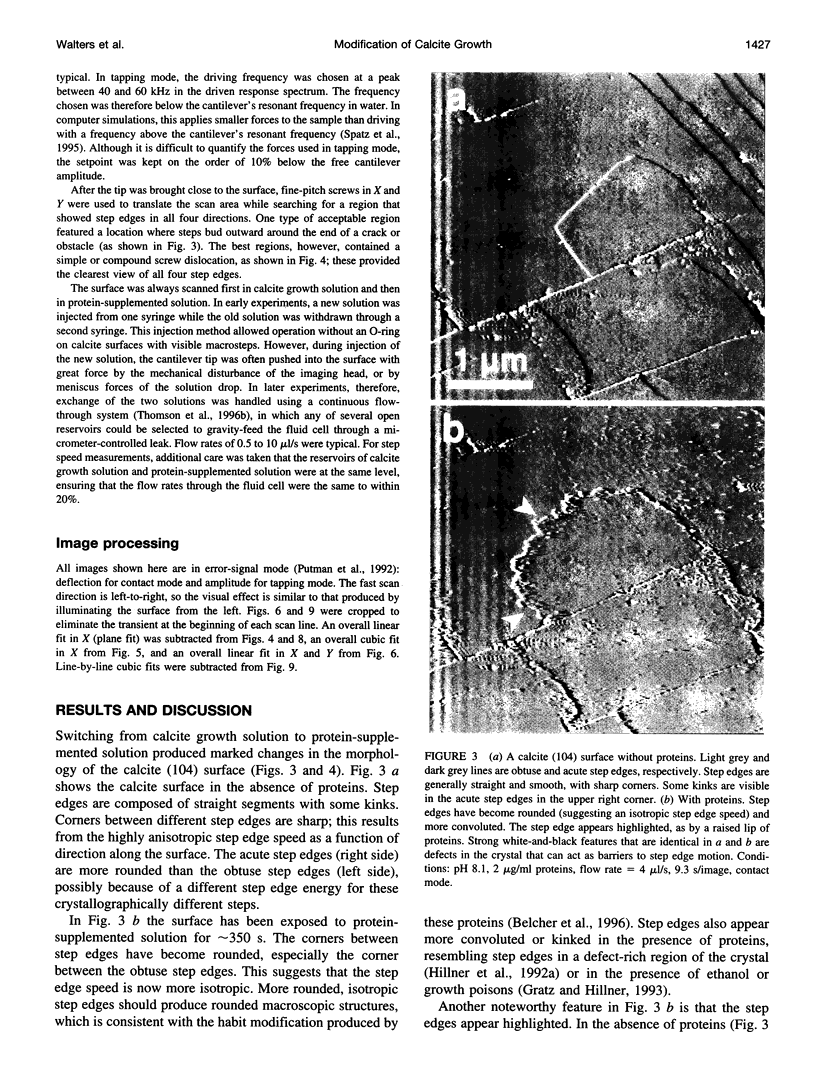
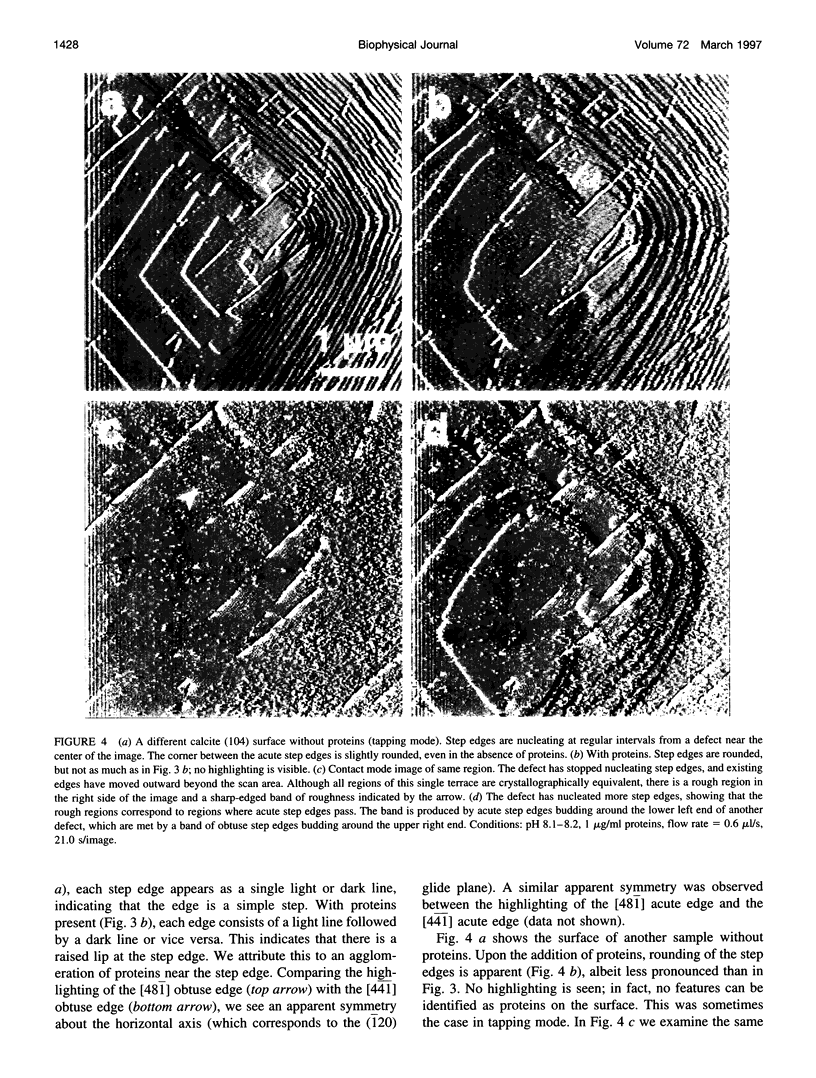
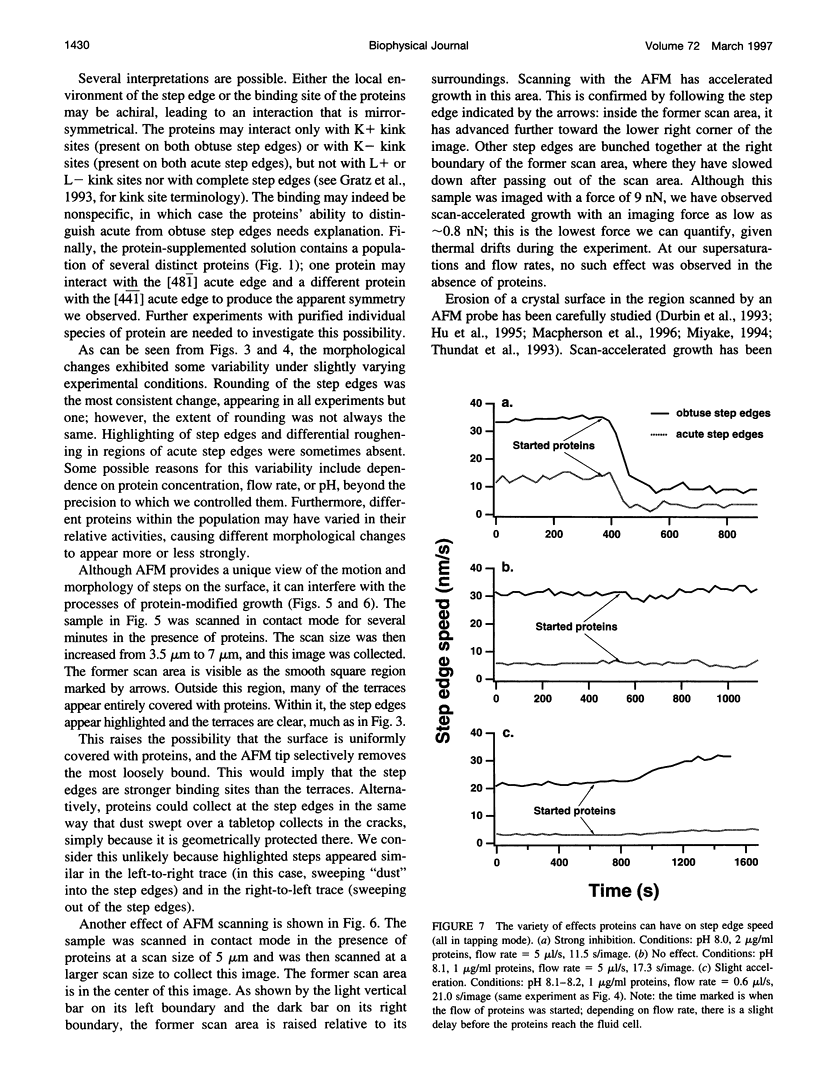
A family of soluble proteins from the shell of Haliotis rufescens was introduced over a growing calcite crystal being scanned in situ by an atomic force microscope (AFM). Atomic step edges on the crystal surface were altered in shape and speed of growth by the proteins. Proteins attached nonuniformly to the surface, indicating different interactions with crystallographically different step edges. The observed changes were consistent with the habit modification induced by this family of proteins, as previously observed by optical microscopy. To facilitate further studies in this area, AFM techniques and certain AFM imaging artifacts are discussed in detail.
Full text
PDF


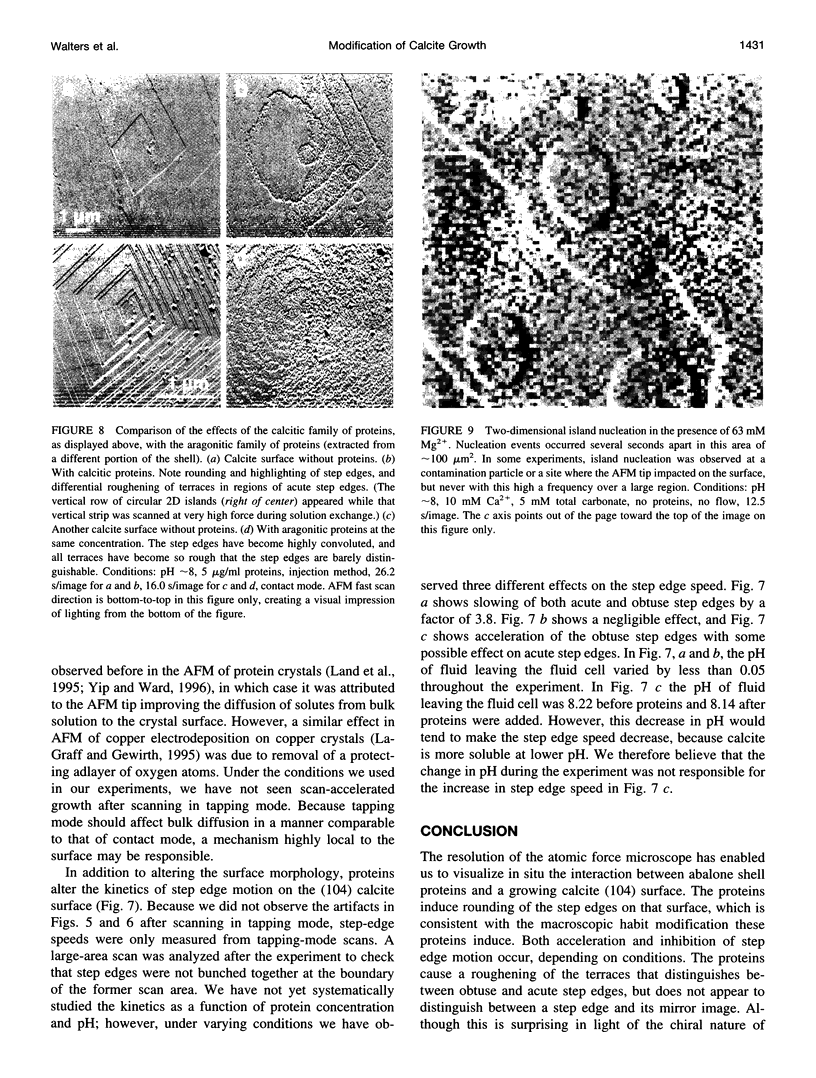


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berman A., Hanson J., Leiserowitz L., Koetzle T. F., Weiner S., Addadi L. Biological control of crystal texture: a widespread strategy for adapting crystal properties to function. Science. 1993 Feb 5;259(5096):776–779. doi: 10.1126/science.259.5096.776. [DOI] [PubMed] [Google Scholar]
- Cleveland JP, Schäffer TE, Hansma PK. Probing oscillatory hydration potentials using thermal-mechanical noise in an atomic-force microscope. Phys Rev B Condens Matter. 1995 Sep 15;52(12):R8692–R8695. doi: 10.1103/physrevb.52.r8692. [DOI] [PubMed] [Google Scholar]
- Dussol B., Geider S., Lilova A., Léonetti F., Dupuy P., Daudon M., Berland Y., Dagorn J. C., Verdier J. M. Analysis of the soluble organic matrix of five morphologically different kidney stones. Evidence for a specific role of albumin in the constitution of the stone protein matrix. Urol Res. 1995;23(1):45–51. doi: 10.1007/BF00298850. [DOI] [PubMed] [Google Scholar]
- Hansma H. G., Laney D. E., Bezanilla M., Sinsheimer R. L., Hansma P. K. Applications for atomic force microscopy of DNA. Biophys J. 1995 May;68(5):1672–1677. doi: 10.1016/S0006-3495(95)80343-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land TA, Malkin AJ, Kuznetsov YG, McPherson A, De Yoreo JJ Mechanisms of protein crystal growth: An atomic force microscopy study of canavalin crystallization. Phys Rev Lett. 1995 Oct 2;75(14):2774–2777. doi: 10.1103/PhysRevLett.75.2774. [DOI] [PubMed] [Google Scholar]
- Malkin A. J., Kuznetsov YuG, Land T. A., DeYoreo J. J., McPherson A. Mechanisms of growth for protein and virus crystals. Nat Struct Biol. 1995 Nov;2(11):956–959. doi: 10.1038/nsb1195-956. [DOI] [PubMed] [Google Scholar]
- Thomson N. H., Fritz M., Radmacher M., Cleveland J. P., Schmidt C. F., Hansma P. K. Protein tracking and detection of protein motion using atomic force microscopy. Biophys J. 1996 May;70(5):2421–2431. doi: 10.1016/S0006-3495(96)79812-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh D., Hopwood J. D., Mann S. Crystal tectonics: construction of reticulated calcium phosphate frameworks in bicontinuous reverse microemulsions. Science. 1994 Jun 10;264(5165):1576–1578. doi: 10.1126/science.264.5165.1576. [DOI] [PubMed] [Google Scholar]
- Wierzbicki A., Sikes C. S., Madura J. D., Drake B. Atomic force microscopy and molecular modeling of protein and peptide binding to calcite. Calcif Tissue Int. 1994 Feb;54(2):133–141. doi: 10.1007/BF00296064. [DOI] [PubMed] [Google Scholar]
- Worcester E. M. Urinary calcium oxalate crystal growth inhibitors. J Am Soc Nephrol. 1994 Nov;5(5 Suppl 1):S46–S53. doi: 10.1681/ASN.V55s46. [DOI] [PubMed] [Google Scholar]
- Yip C. M., Ward M. D. Atomic force microscopy of insulin single crystals: direct visualization of molecules and crystal growth. Biophys J. 1996 Aug;71(2):1071–1078. doi: 10.1016/S0006-3495(96)79307-4. [DOI] [PMC free article] [PubMed] [Google Scholar]