Abstract
The Escherichia coli AmtB protein is member of the ubiquitous Amt family of ammonium transporters. Using a variety of [14C]methylammonium-uptake assays in wild-type E. coli, together with amtB and glutamine synthetase (glnA) mutants, we have shown that the filtration method traditionally used to measure [14C]methylammonium uptake actually measures intracellular accumulation of methylglutamine and that the kinetic data deduced from such experiments refer to the activity of glutamine synthetase and not to AmtB. Furthermore, the marked difference between the Km values of glutamine synthetase calculated in vitro and those calculated in vivo from our data suggest that ammonium assimilation by glutamine synthetase is coupled to the function of AmtB. The use of a modified assay technique allows us to measure AmtB activity in vivo. In this way, we have examined the role that AmtB plays in ammonium/methylammonium transport, in the light of conflicting proposals with regard to both the mode of action of Amt proteins and their substrate, i.e. ammonia or ammonium. Our in vivo data suggest that AmtB acts as a slowly conducting channel for NH3 that is neither dependent on the membrane potential nor on ATP. Furthermore, studies on competition between ammonium and methylammonium suggest that AmtB has a binding site for NH4+ on the periplasmic face.
Keywords: ammonium channel, ammonium transport, AmtB, glutamine synthetase, methylammonium, nitrogen metabolism
Abbreviations: Amt, ammonium transporter; CCCP, carbonylcyanide m-chlorophenylhydrazone; Ea, activation energy; GlpF, glycerol facilitator; GlpK, glycerol kinase; GS, glutamine synthetase; MA, methylammonium; Mep, methylammonium permease; MSF, L-methionine sulphone; MSX, L-methionine-S-sulphoximine; Rh, Rhesus; RhAG, Rh-associated glycoprotein
INTRODUCTION
The Amt (ammonium transporter) or Mep (methylammonium permease) proteins form a family of novel membrane proteins that are present in all three domains of life [1]; in animals they are represented by the Rh (Rhesus) proteins [2]. Escherichia coli encodes a single Amt protein, AmtB, encoded in the glnK amtB operon whose expression is almost negligible in ammonium excess, but is induced substantially when ammonium is limiting for growth. Under these conditions, ammonium is assimilated by the combined activities of GS (glutamine synthetase) and glutamate synthase. [Throughout the present paper, we use the term ammonium to refer to both the protonated (NH4+) and the unprotonated (NH3) forms. Chemical symbols are used when the protonation state is important.]
AmtB is a stable trimer in the cytoplasmic membrane and retains this structure when purified and reconstituted in two-dimensional crystals [3,4]. GlnK is a member of the PII family of signal transduction proteins that interacts with AmtB to regulate its activity in response to intracellular nitrogen status [5,6]. This regulation occurs in response to micromolar changes in the extracellular ammonium concentration and is tightly coupled to the intracellular glutamine pool, indicating that AmtB activity and ammonium metabolism are intimately connected [6].
AmtB is essential for uptake of the ammonium analogue, [14C]MA ([14C]methylammonium), in E. coli [7]. However, the application of a variety of experimental approaches has not provided clear evidence for the mechanism of transport into the cell. The prevailing view has been that these systems function in active transport of NH4+, to concentrate it within the cell [8]. Studies of ammonium transport in Corynebacterium glutamicum suggested that Amt proteins are uniporters that take up NH4+ by a membrane-potential-driven mechanism [9]. This proposal was reinforced by electrophysiological experiments in which a Lycopersicon esculentum Amt protein (LeAMT1;1), was expressed in Xenopus oocytes [10]. Both ammonium and MA induced voltage-dependent currents, and the Km for NH4+ was independent of the external pH, arguing that NH4+ is the Amt substrate. Subsequent electrophysiological studies of the Rh protein RhBG suggested that this protein also mediates NH4+ transport, either as an electroneutral NH4+/H+ exchanger [11] or as an electrogenic NH4+ transporter [12].
An alternative model was proposed by Kustu and co-workers who investigated the roles of E. coli AmtB and the Saccharomyces cerevisiae Mep proteins and concluded that these proteins facilitate diffusion of ammonia (NH3) across the cytoplasmic membrane [13,14]. The X-ray crystallographic structure of E. coli AmtB has now been resolved, and structures with and without bound ammonia or methylammonia indicate that the protein does indeed have the characteristics of a channel that conducts NH3 [15,16]. A similar conclusion was reached using stopped-flow studies of facilitated NH3 movement across the erythrocyte membrane by RhAG (Rh-associated glycoprotein) [17].
In the present paper, we report the application of a variety of assays to address the mode of action of E. coli AmtB in vivo. Our data provide insight into the conflicting in vivo data reported previously. They suggest that the flux of ammonia through AmtB may be closely coupled to its subsequent metabolism by GS and they show that the channel model proposed from analysis of the AmtB structure is consistent with in vivo data.
EXPERIMENTAL
Strains, plasmids and culture conditions
Strains and plasmids used in the present study are listed in Table 1. Strain GT1004 is a ΔamtB derivative of ET8004 constructed in an identical manner with GT1001 [7]. For growth of E. coli under nitrogen limitation, an altered M9 medium [18] was used, in which ammonium sulphate was replaced by 200 μg·ml−1 glutamine (M9Gln medium). Cells were grown overnight at 30 °C, using 10 ml of growth medium in a 50 ml conical flask, shaken at 250 rev./min. Chloramphenicol was used at 15 μg·ml−1 and ampicillin at 100 μg·ml−1.
Table 1. Strains and plasmids.
| Strain/plasmid | Genotype or phenotype | Reference/source |
|---|---|---|
| E. coli strains | ||
| ET8000 | rbs lacZ::IS1 gyrA hutCK | [40] |
| ET8004 | rbs lacZ::IS1 gyrA hutCKglnA1885 | [41] |
| GT1000 | ET8000 ΔglnK amtB | [5] |
| GT1001 | ET8000 ΔamtB | [5] |
| GT1002 | ET8000 ΔglnK | [5] |
| GT1004 | ET8000 ΔamtB glnA1885 | Present study |
| S. cerevisiae strains | ||
| 23344c | MATα, ura3 | [20] |
| 31019b | MATa, ura3, mep1Δ, mep2Δ, mep3Δ | [20] |
| Plasmids | ||
| pMM286 | amtB cloned into p416MET25 | Present study |
| pMM288 | amtB cloned from pMM286 into p426MET25 | Present study |
| p416MET25 | Low-copy yeast expression vector | [42] |
| p426MET25 | High-copy yeast expression vector | [42] |
Plasmid construction
To enable expression in yeast, amtB was amplified by PCR from pGC2 with the following primers: AMT7, 5′-GCTCTAGACATATGAAGATAGCGACGATA-3′, and AMT2, 5′-CCGGATCCTTACAGATCTGCGTTATAGGCATTCTCGCC-3′, thereby introducing an XbaI site at the 5′ end of the gene and a BamHI site after the stop codon. The XbaI/BamHI-cut PCR product was cloned into the XbaI/BamHI sites of the low-copy yeast shuttle vector p416MET25 forming pMM286. From this, a SacI/HindIII fragment was cloned into a high-copy-number derivative, p426MET25, creating pMM288.
[14C]MA transport assays
MA uptake and assimilation rates were determined essentially as described previously [7]. This methodology is subsequently referred to as a ‘washed’ assay. Radioactivity in the samples was counted with a Wallac 1409 scintillation counter, which had been calibrated with a known set of standards.
Unwashed assays were modified from the method of Jayakumar and Barnes [19]. Cells were prepared as for washed assays, and 80 μl samples were spotted on to a double filter consisting of a polycarbonate filter overlaid on two Whatman filter paper disks. Samples were filtered to dryness within 1 s. Radioactivity on the polycarbonate filter was measured using scintillation counting essentially as described previously [7]. Unless otherwise stated, both washed and unwashed assays were performed at 22 °C.
RESULTS
E. coli AmtB can transport ammonium
Although AmtB is essential for [14C]MA uptake, deletion of the amtB gene does not result in detectable growth impairment on low concentrations of ammonium at pH 7.0 [7,13], whereas, in S. cerevisiae, deletion of all three mep genes renders cells unable to grow on less than 5 mM ammonium [20]. A number of eukaryotic Amt proteins can complement growth of the S. cerevisiae triple mep mutant. We confirmed that E. coli AmtB is capable of transporting ammonium (either as NH4+ or as NH3) by cloning amtB into a yeast vector and transforming this plasmid (pMM288) into a triple mep mutant. This construct grew on minimal medium agar plates buffered at pH 6.1 and containing 1 mM ammonium, conditions in which 99% of the ammonium is present as NH4+ (see Supplementary Figure 1S at http://www.BiochemJ.org/bj/390/bj3900215add.htm).
Use of washed compared with ‘unwashed’ assays for MA transport
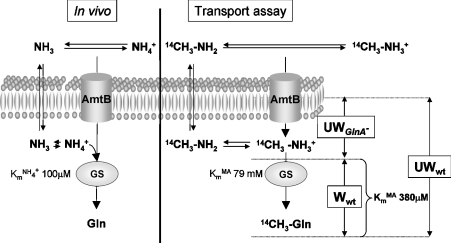
In the [14C]MA-uptake assay that has been used almost universally to assess Amt activity in vivo, free intracellular MA is removed from cells when the filters are washed. Consequently the radioactivity measured is only from metabolized products of MA [19]. In many bacteria, including E. coli, [14C]MA is converted by GS into [14C]methylglutamine, accumulation of which can be mistaken for concentrative MA uptake. Consequently, the kinetics derived from these assays compound data for [14C]MA uptake and for its subsequent assimilation by GS (Figure 1).
Figure 1. Model comparing ammonium uptake and assimilation in vivo with those components measured in MA-uptake assays.
Using a protocol without filter washing, it is possible to measure specifically the MA accumulation in a glnA− strain and MA plus methylglutamine accumulation in the wild-type (wt) strain. Using a protocol with filter washing, only methylglutamine accumulation is measured. W, washed assay; UW, unwashed assay.
Using this assay in E. coli, Soupene et al. [13] saw no accumulation of MA in a GS mutant, and concluded that AmtB does not concentrate MA and that the transport is a passive process. However Jayakumar and Barnes [19] developed an assay that does not require a washing step, and allows determination of both free and metabolized intracellular MA. Using this unwashed assay with a GS mutant allows measurement of [14C]MA uptake without its subsequent assimilation. The application of both the washed and unwashed assays has allowed us to analyse AmtB-dependent MA uptake in E. coli and thereby to clarify the interpretation of previous data.
AmtB-dependent uptake of [14C]MA can be separated from assimilation
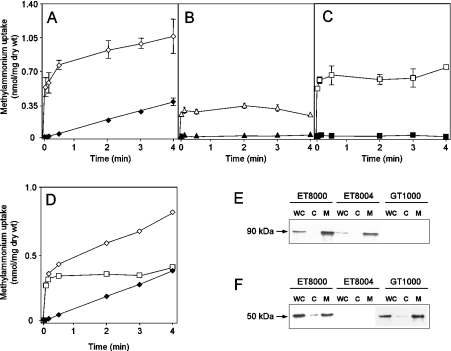
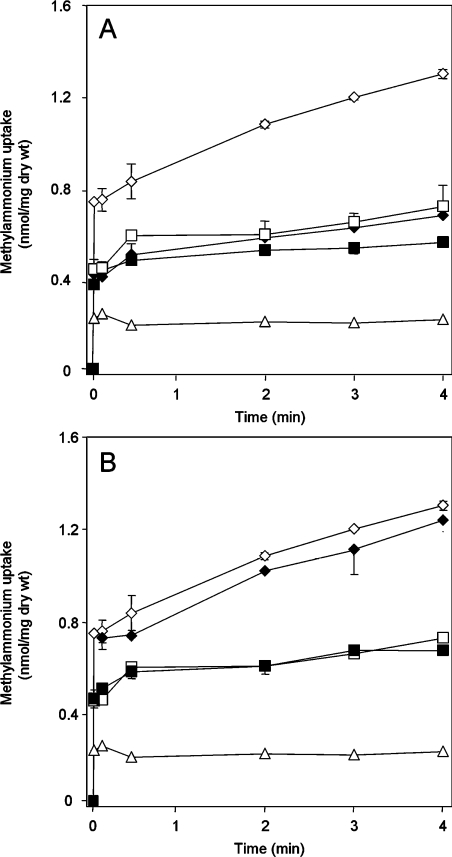
We confirmed findings of a previous study [19] that, in the unwashed assays, only a small fraction of the radioactivity added to the filter was retained in the absence of washing. We obtained a value of less than 1% retention of radiolabel under all conditions tested. We then compared MA uptake with and without washing in the wild-type strain, ET8000, using time points of 5, 10, 30, 120, 180 and 240 s. There was a highly significant difference in the initial rate of uptake measured in washed or unwashed assays, but, after 30 s, the rates of uptake were similar under both conditions (Figure 2A).
Figure 2. Time course of [14C]MA influx using washed versus unwashed assays.
[14C]MA transport activities were measured by adding 20 μM (final concentration) of [14C]MA at zero time. (A) ET8000 (wild-type), (B) GT1000 (glnK− amtB−), (C) ET8004 (glnA−). (D) ET8000 (◇) and ET8004 (□) minus the GT1000 uptake rate. Open symbols, unwashed assays; closed symbols, washed assays. Values are means±S.D. (n=6). (E) Whole-cell extracts (WC), cytoplasmic (C) and membrane (M) fractions were subjected to SDS/PAGE, followed by Western blotting using an anti-AmtB antibody. Each lane was loaded with 5 μg of protein, but the membrane fraction was concentrated ×20 compared with the whole-cell extract. (F) as (E), but using an anti-GS antibody. Extracts were prepared from ET8000 (wild-type), GT1000 (glnK− amtB−) and ET8004 (glnA−) grown in M9Gln medium.
We hypothesized that the rapid phase of uptake in the unwashed cells (compared with the washed samples which effectively measure what has been assimilated) is due to the activity of AmtB. To test this hypothesis, we repeated the experiment with the following isogenic strains: glnK amtB deletion strain GT1000, amtB deletion strain GT1001, glnA (GS−) strain ET8004 and the amtB glnA deletion strain GT1004. The levels of AmtB in ET8000 and ET8004 were shown to be equivalent by Western blotting (Figure 2E). The intracellular levels of GS in ET8000 and GT1000 were also shown to be equivalent by Western blotting, and a significant proportion of GS was membrane-associated in an AmtB-independent fashion (Figure 2F).
The initial rapid uptake of MA in the wild-type strain is not seen in GT1000 (Figure 2B), or in GT1001 and GT1004 (results not shown). In ET8004, which lacks GS and therefore cannot convert [14C]MA into [14C]methylglutamine, an initial rapid uptake occurs that is identical with that seen in the wild-type strain, but, after 30 s, the intracellular level of [14C]MA remains constant (Figure 2C). Although there is an increase in radioactivity counts in the absence of washing (Figure 2B), this is probably due to non-specific association with the biomass. If it was due to diffusion of methylamine across the membrane, it should increase with increasing external pH, but we saw no change when the experiment was carried out at various external pH values (results not shown). We could detect methylamine diffusion by measuring the trapped methyglutamine in a washed assay of an amtB mutant (GT1000) at various pHs. However, the levels are negligible, amounting to only 3% of the total under these conditions (compare Figures 2B and 7A). AmtB-independent accumulation of MA can become significant at very high, but non-physiological, external MA concentrations, such that, at 2 mM MA, this accounts for approx. 30% of total MA assimilation (see Figure 4).
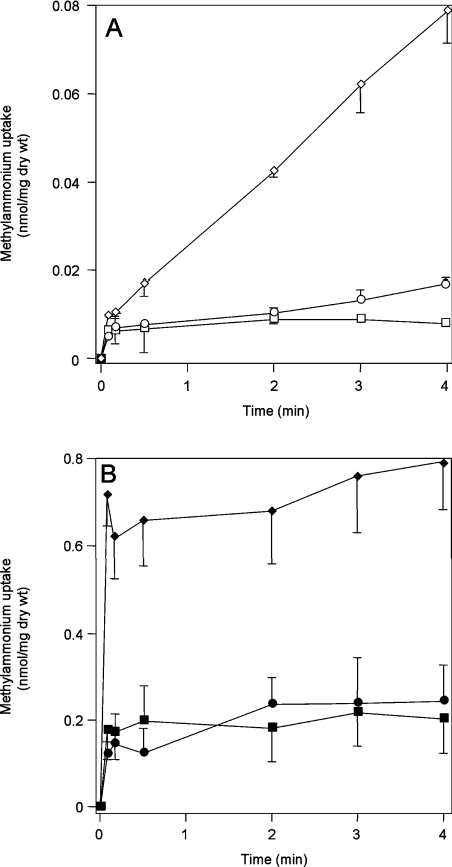
Figure 7. Effect of pH on [14C]methylglutamine accumulation and [14C]MA uptake.
(A) [14C]Methylglutamine accumulation measured in using washed assays in GT1000 (glnK− amtB−) at pH 6.25 (□), 7.25 (○) and 8.25 (◇). (B) [14C]MA uptake measured using unwashed assays in ET8004 (amtB−) at pH 6.25 (■), 7.25 (●) and 8.25 (◆). Cells were prepared as described in the Experimental section, but re-suspended in the final M9 minimal medium buffered at the desired pH using 0.2 M Tris/maleate buffer.
Figure 4. In vivo kinetics of GS activity.
GS activity was measured by calculating the slope of the curve for [14C]methylglutamine accumulation in washed assays from time points at 30 s, and 2, 3 and 4 min over a [14C]MA concentration range of 10 μM to 2 mM. Accumulation was measured in GT1000 (●) and ET8000 (○). Data was fitted to Michaelis–Menten kinetics using Prism version 3.02 software (GraphPad).
For clarification, we subtracted the data for GT1000 from that for ET8000 and ET8004, thereby obtaining a direct comparison of AmtB-dependent uptake in the presence or absence of GS (Figure 2D). Initial MA uptake is equivalent in wild-type and glnA− strains and therefore reflects AmtB-dependent movement of MA across the membrane (see Figure 1). After 30 s, the MA level in the glnA− strain does not change, whereas, in the wild-type strain, accumulation continues at a linear rate, indicating conversion of MA into methylglutamine. Identical rates of methylglutamine accumulation are seen in both the washed [0.093 nmol·min−1·(mg of dry weight)−1] and unwashed [0.086 nmol·min−1·(mg of dry weight)−1] assays, consistent with the interpretation that this accumulation reflects the activity of GS in both cases (Figure 2D).
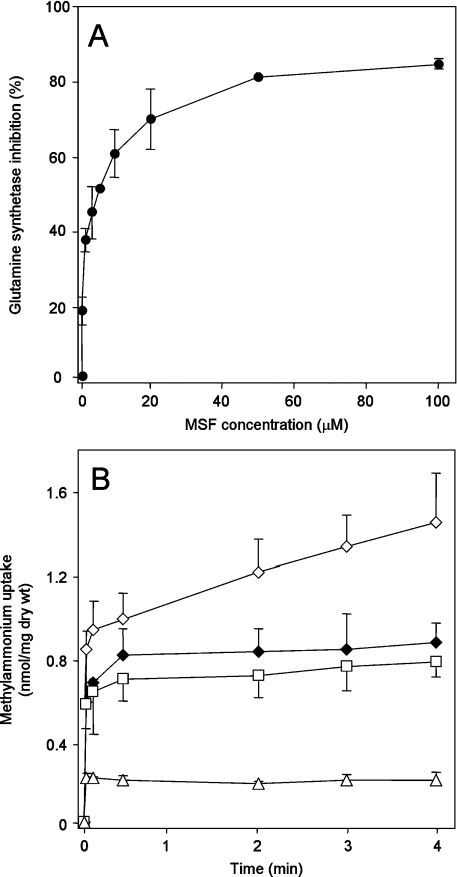
An independent demonstration of the role of GS can be provided by the use of GS inhibitors. The inhibitors MSX (L-methionine-S-sulphoximine) and MSF (L-methionine sulphone) are analogues of the tetrahedral intermediate formed in the course of glutamine synthesis by the reaction of ammonia with enzyme-bound γ-glutamyl phosphate. We found that MSF is a much more effective inhibitor of E. coli GS activity in vivo than MSX (results not shown). In washed assays, methylglutamine accumulation was progressively inhibited with increasing MSF concentration, such that the maximum inhibition (approx. 80%) was achieved at above 20 μM MSF (Figure 3A). In unwashed assays, 20 μM MSF produced an identical accumulation curve in the wild-type (ET8000) to that seen in the glnA strain ET8004 in the absence of MSF (Figure 3B). Addition of 20 μM MSF to ET8004 or GT1000 had no effect. These results substantiate the proposition that MA uptake occurs in two phases, the first of which is AmtB-dependent and the second of which is a consequence of GS activity.
Figure 3. Effects of the GS inhibitor MSF on [14C]MA uptake.
(A) Inhibition of GS by MSF. GS activity was measured by calculating the slope of the curve for [14C]methylglutamine accumulation in washed assays from time points at 30, 120, 180 and 240 s with or without MSF pre-treatments. MSF (1–100 μM) was added to cultures 4 min before starting the transport assays by adding [14C]MA. Results are expressed as percentage inhibition compared with the activity in the absence of MSF, and are means±S.D. (n=4). (B) Effect of MSF on MA uptake measured in unwashed assays. ET8000 (wild-type) without pre-treatment (◇), or pre-treated for 4 min with 20 μM MSF (◆), ET8004 (glnA−) (□), GT1000 (glnK− amtB−) (△). Results are means±S.D. (n=4).
Kinetics of [14C]methylglutamine accumulation
Previous studies of GS have been either in vitro with the purified enzyme [21] or in vivo using E. coli cells treated with the detergent hexadexyltrimethylammonium bromide to render the cells permeable to the reactants [22]. Using washed assays, we are able, for the first time, to assess the in vivo activity of GS without permeablizing the cells. We determined rates of [14C]methylglutamine accumulation for ET8000 and GT1000 grown at pH 7.25, over a substrate concentration range of 10 μM to 2 mM. We confirmed that, even at 2 mM [14C]MA, the washed assay measures only [14C]methylglutamine accumulation, and not methylamine diffusion across the membrane, because the level of 14C measured in ET8004 does not increase from 10 μM to 2 mM [14C]MA (results not shown). The GS activity measured in ET8000 is saturable and follows Michaelis–Menten kinetics (Figure 4). From a Hanes–Woolf plot of the data, we determined an apparent Vmax of 1.5 nmol·min−1·(mg of dry weight)−1 and a Km of 380 μM. In further support of the argument that the washed assay measures GS activity, the Arrhenius plot for data from a washed assay was linear from 4 to 37 °C with an Ea (activation energy) of 14.1 kcal·mol−1 (see Supplementary Figure 2S at http://www.BiochemJ.org/bj/390/bj3900215add.htm). This may be compared with a previous independent in vitro calculation of the Ea for E. coli GS of 18.3 kcal·mol−1 [23].
The linear kinetics observed for MA assimilation in GT1000 (Figure 4) could be due to methylamine diffusion across the membrane and its subsequent assimilation by GS, or to protein-mediated, AmtB-independent MA accumulation. This process accounts for 3% (at 20 μM MA) to 30% (at 2 mM MA) of total MA assimilated.
AmtB acts as an NH3 channel
The unwashed assays shown in Figure 2(D) used a starting external concentration of 20 μM MA at pH 7.25, and, in the absence of GS, the internal MA concentration reached a steady state level of 0.3 nmol·min−1·(mg of dry weight)−1 within 30 s. Using ET8004 and GT1000, we investigated how this internal steady-state MA level changed when the external concentration of MA was varied. Both strains showed a completely linear dependence over a range of external MA concentrations from 10 μM to 2 mM (Figure 5). As discussed above, MA accumulation in GT1000 is due to a combination of non-specific association with the biomass, diffusion, and AmtB-independent accumulation. The subtraction of these data from those for ET8004 (Figure 5) indicates that the internal MA concentration reaches an AmtB-dependent equilibrium within 30 s of the addition of MA to the external medium in a manner that is completely consistent with AmtB acting as a channel.
Figure 5. Kinetics of [14C]MA uptake based on unwashed assays.
The AmtB-dependent accumulation of [14C]MA at equilibrium was determined by calculating the mean level from time points at 30, 120, 180 and 240 s in ET8004 (glnA−) (□), GT1000 (glnK− amtB−) (△), and data were derived after subtraction of accumulation measured in GT1000 from that measured in ET8004 (○). A concentration range of 10 μM to 2 mM MA was used with the unwashed protocol. Results are means±S.D. (n=4).
Membrane proteins that function as channels rather than as transporters can also be readily distinguished by their Ea. Channel proteins such as aquaporins have an Ea of <5 kcal·mol−1 [24], whereas the Ea of transporters fall in the range 11–27 kcal·mol−1 [25]. Furthermore, the Ea for NH3 permeability through a cell membrane is also relatively high at 11.7 kcal·mol−1 [26]. Using unwashed assays, we determined the Ea for AmtB activity. The Arrhenius plot was linear from 4 to 37 °C with an Ea of 1.6 kcal·mol−1 (see Supplementary Figure 2S at http://www.BiochemJ.org/bj/390/bj3900215add.htm), strongly supporting the proposition that AmtB functions as a channel.
Using estimates of the cell volume of 1 μm3 (measurements determined from electron microscopy of thin sections of cells grown in M9Gln medium) and of cellular dry weight of 3.6×10−13 g·cell−1, we calculated a cell volume of approx. 3 μl·(mg of cellular dry weight)−1. The data from Figure 5 (mean from 12 external MA concentrations) was then used to calculate the relative accumulation (internal/external), giving a ratio of 3.9±0.6. In principle, if AmtB acts as a channel through which NH4+ (or the analogue [14C]MA in our experiments) is driven by the membrane potential ΔΨ, then, with a ΔΨ of 140 mV [27], we would expect a substrate concentration gradient of 200-fold. Such a concentrative effect is not observed (indeed the calculated ratio is relatively close to unity), and our in vivo data are therefore consistent with the model from the X-ray structure of AmtB [15,16], namely that the species conducted by AmtB is NH3.
AmtB activity is independent of the membrane potential and of ATP
Previous studies using protonophores, such as CCCP (carbonylcyanide m-chlorophenylhydrazone) or 2,4-dinitrophenol have demonstrated inhibition of MA uptake in washed assays and have concluded that Amt activity is dependent on the ΔΨ [9,28,29]. In E. coli, CCCP has pleiotropic effects on cellular metabolism, such that although 5 μM CCCP is sufficient to completely dissipate the ΔΨ, 50 μM CCCP causes a fall in the intracellular ATP pools to 35–50% of that in untreated cells within 45 s of addition [27]. Using washed assays, methylglutamine accumulation was inhibited almost completely by addition of 40 μM CCCP. In unwashed assays, 40 μM CCCP produced an identical accumulation curve in the wild-type strain with that seen in the glnA− strain ET8004 in the absence of CCCP (Figure 6A). These data are consistent with an indirect effect of CCCP on GS activity, as a consequence of lowering the ATP pool, and demonstrate that AmtB-dependent uptake is not dependent on ATP. However, 10 μM CCCP, which should completely dissipate the ΔΨ, had no effect on GS activity, as measured by methylglutamine accumulation in washed or unwashed assays, nor on AmtB-dependent MA-uptake activity in both ET8000 and ET8004 (Figure 6B). These data substantiate the proposal that E. coli AmtB function is independent of ΔΨ.
Figure 6. Effects of the protonophore CCCP on [14C]MA uptake.
Unwashed assays in which CCCP, at (A) 40 μM or (B) 10 μM, was added to cultures 3 min before the addition of [14C]MA. ET8000 (wild-type) without pre-treatment (◇), or pre-treated with CCCP (◆), ET8004 (glnA−) without pre-treatment (□), or pre-treated with CCCP (■), GT1000 (glnK− amtB−) (△). Results are means±S.D. (n=4).
pH-dependence of MA uptake by AmtB
The pKa for MA/methylamine is 10.25 and, consequently, availability of methylamine varies strongly with pH, such that an increase of 1 pH unit will lead to a 10-fold increase in the methylamine concentration. We therefore investigated how AmtB-dependent MA accumulation varied if the external pH was 6.25, 7.25 or 8.25.
Initially, we measured MA assimilation in an amtB− mutant (GT1000). The rate of MA assimilation increased by a factor of 2.7 between pH 6.25 and 7.25 and a factor of 6.4 between pH 7.25 and 8.25 (Figure 7A). Hence, we do observe a pH-related increase, but not of the theoretically expected magnitude. Such discrepancies with respect to the movement of ammonia across membranes have been observed in other systems [26] (see the Discussion). We then used unwashed assays to determine the pH-dependence of AmtB activity at the same three pH values. There was no significant difference in MA equilibration between pH 6.25 and 7.25, and the equilibration level increased by only a factor of 3.8 between pH 7.25 and 8.25 (Figure 7B). Hence, the intracellular AmtB-dependent MA accumulation does not depend only on the concentration of the uncharged species in the bulk solution as implied by Soupene et al. [13].
Inhibition of MA uptake by ammonium
In previous studies using washed assays of [14C]MA uptake by a variety of Amt proteins, inhibition by ammonium has often been used to determine a Ki for ammonium that has been interpreted as being equivalent to the Km of the relevant Amt protein for ammonium [20]. We have now shown that such an approach in E. coli would actually measure the Ki for ammonium of GS and, consequently, inhibition studies of AmtB require the use of unwashed assays.
Data from unwashed assays at 22 °C suggested that a 5 s time point is just within the linear phase of AmtB-dependent MA uptake (Figure 2), but, to examine this further, we carried out washed and unwashed assays at 7 and 16 °C in strain ET8004 (see Supplementary Figure 3S at http://www.BiochemJ.org/bj/390/bj3900215add.htm). The time to reach equilibration of MA varied inversely with the temperature, and it was apparent that at 7 °C we see true kinetics up to 30 s; at 16 °C, the 10 s time point is within the uptake kinetics, and therefore at 22 °C, the 5 s time point is a reasonable measure for the initial uptake.
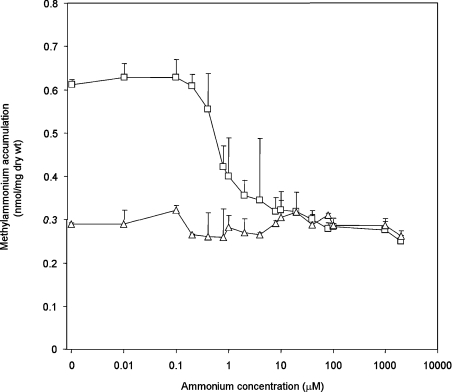
Using 20 μM [14C]MA, we determined the amount of [14C]MA accumulated after 5 s and the effects of this accumulation on the coincident presence of ammonium at concentrations varying from 0.01 μM to 2 mM. For each condition, we compared ET8004 and GT1000. [14C]MA uptake was inhibited significantly at 0.4 μM ammonium and, with 8 μM ammonium, the uptake level was equivalent to that in GT1000 (Figure 8). The ammonium-inhibition profile in ET8004 was not significantly different from that in ET8000 or the glnK− strain, GT1002 (results not shown). Hence, MA equilibration by AmtB is significantly inhibited by ammonium, suggesting that the two substrates compete for initial binding to the periplasmic face of the protein.
Figure 8. Inhibition of [14C]MA accumulation by ammonium in unwashed assays.
The level of [14C]MA accumulation was measured 5 s after addition of the substrate. [14C]MA (20 μM) was used with ammonium (0.01 μM to 2000 μM) added at zero time. ET8004 (glnA−) (□), GT1000 (glnK− amtB−) (△). Results are means±S.D. (n=4).
DISCUSSION
AmtB function is coupled to ammonium assimilation
It has been recognized by some authors that the in vivo analysis of Amt-mediated transport of the ammonium analogue [14C]MA using washed filter assays potentially compounds the processes of transport and metabolism [13,28]. Nevertheless, the unwashed assay protocol devised by Jayakumar and Barnes [19] has not been widely adopted. This technique allows direct measurement of the kinetics of MA uptake through AmtB and its separation from assimilation by GS. We have now demonstrated that, in E. coli (and, by analogy, potentially in other bacteria), the washed assay of [14C]MA uptake measures [14C]MA assimilation by GS and consequently kinetic parameters determined in this way do not relate to AmtB.
Previous studies examined the in vitro affinity of GS from E. coli and Azotobacter vinelandii for both MA and ammonium [21,30–32]. The Km of 380 μM determined in our studies is the effective in vivo Km of E. coli GS for MA and the equivalent in vivo Km for A. vinelandii GS was calculated previously [33]. When these data are compared, it is very apparent that the results determined independently for the two organisms are remarkably similar and that there is a very large difference between the Kms of GS for MA in vivo and in vitro in both organisms (Table 2). Furthermore, there is an approx. 1000-fold difference between the in vitro Km of the enzyme for ammonium and MA. The in vivo Km of GS for ammonium has not been determined and, as mentioned above, such studies have traditionally utilized permeabilized cells [22].
Table 2. Km values for GS.
In summary, the substrate affinity of GS is significantly greater when determined in vivo than when calculated in vitro using the purified enzyme, and a possible explanation for this discrepancy is that the function of GS can, at least in some circumstances, be closely coupled to that of AmtB. In keeping with this concept, we have shown that, in E. coli, significant amounts of GS are membrane-bound, although in an AmtB-independent fashion (Figure 2), and, in A. vinelandii, 30% of GS activity was found to be membrane-associated [32].
This proposed mechanism of AmtB action is reminiscent of the glycerol channel GlpF (glycerol facilitator). Although the channel properties of GlpF can be characterized when the protein is heterologously expressed in Xenopus oocytes [34], in vivo GlpF functions in concert with GlpK (glycerol kinase) which traps glycerol inside the cell as glycerol 3-phosphate [35]. In vivo studies suggest that GlpK may interact with GlpF to facilitate glycerol transport, although no interaction between the two proteins has yet been demonstrated [35].
In principle, if metabolic coupling of AmtB to GS occurred, we might expect that there would be little or no free ammonium pool in the cell and hence no significant difference between the uptake profile in washed and unwashed assays. However, it is important to note that the assays reported here all employ the ammonium analogue MA rather than ammonium itself. MA is a poor substrate for GS and our ammonium-inhibition data indicate that it is also less effective than ammonium as a substrate for AmtB. Consequently, MA is a poor analogue for ammonium both biochemically and physiologically.
The use of [14C]MA is also non-physiological in other respects. Post-translational inhibition of AmtB activity is mediated by interaction with GlnK and occurs in response to external ammonium concentrations of 10–100 μM, consistent with a role for AmtB in scavenging low concentrations of ammonium [6]. Consequently, AmtB is unlikely to facilitate ammonium uptake at concentrations above ∼100 μM. However, unlike ammonium, MA does not promote deuridylylation of GlnK (A. Javelle and M. Merrick, unpublished work) and, consequently, high levels of MA do not lead to GlnK-dependent inactivation of AmtB. As a result, we can measure [14C]MA uptake at external concentrations of 2 mM or more (see Figure 4).
AmtB functions as a channel
A number of different experimental approaches reported in the present paper indicate that E. coli AmtB acts in vivo as a channel. The intracellular MA concentration reaches an AmtB-dependent equilibrium within 30 s of the addition of MA to the external medium. AmtB function is independent of the ΔΨ and of the intracellular ATP pool. The Ea of AmtB is characteristic of a channel, not a transporter, and is similar to that of aquaporins.
Our experiments using different external pH conditions show that AmtB does not thermodynamically equilibrate NH3 between the extracellular bulk solution and the cytoplasm as implied by Soupene et al. [13]. It is well recognized that adjacent to the membrane is a so called ‘unstirred’ layer that acts as a diffusion barrier and in which the chemical conditions deviate substantially from conditions in the bulk extracellular solution [36,37]. As a consequence, pH changes in the bulk solution are not directly reflected in the immediate vicinity of the membrane. We observed a marked increase in MA equilibration when the extracellular pH was increased from pH 7.25 to 8.25, but not of the magnitude expected if this were to reflect the concentration of the uncharged species in the bulk solution. Very similar phenomena have been reported by others studying the permeability of lipid bilayer membranes to a variety of weak bases, including ammonia [26,36]. We therefore suggest that our in vivo data are compatible with the model derived from the X-ray crystal structures of AmtB in which NH4+ is bound in the periplasmic vestibule and deprotonated before the equilibration of NH3 through the channel [15,16]. If ammonia conduction involves binding of NH4+ followed by subsequent deprotonation, then ammonium might be expected to compete with MA and to inhibit MA uptake [15], and indeed our data (Figure 8) confirm that such competition does occur at approx. 1 μM ammonium.
Although it is attractive to extend the channel model for AmtB to other members of the Amt/Rh family [15], this remains to be proven, although recent studies have shown that RhAG also acts as an NH3 channel [17]. Current data on other systems are inconclusive, but those studies may not be discrepant because, as shown by the ClC protein family, the same molecular architecture can support either a channel or an active transport mechanism. The eukaryotic ClC-0, 1 and 2 proteins are Cl−-selective channels, while E. coli ClC-ec1 is a secondary active transporter [38].
Based on a variety of in vivo data, Zheng et al. [16] estimated that AmtB might move ammonia at a rate of only 10–10000 molecules/s per channel compared with the rates of 108–109 ions or molecules/s that typify ion channels that operate close to the diffusion limit. Our in vivo data are consistent with AmtB acting as a slowly conducting channel, such that, at 22 °C, equilibration of MA takes a number of seconds and, at 7 °C, it takes up to 2 min (see Supplementary Figure 3S at http://www.BiochemJ.org/bj/390/bj3900215add.htm). Furthermore, the fact that the protein apparently contains a binding site for NH4+ would predictably reduce the turnover to a few events per s [39].
Acknowledgments
A.J., G.T. and M.M. acknowledge support from the Biotechnology and Biological Sciences Research Council (U.K.). We thank Dan Blakey for construction of plasmids pMM286 and pMM288, and Pierre Ripoche and Yves Colin for helpful discussion. Anne Durand is thanked for critical comments on the paper.
References
- 1.von Wirén N., Merrick M. Regulation and function of ammonium carriers in bacteria, fungi and plants. Trends Curr. Genet. 2004;9:95–120. [Google Scholar]
- 2.Liu Z., Peng J., Mo R., Hui C., Huang C. H. Rh type B glycoprotein is a new member of the Rh superfamily and a putative ammonia transporter in mammals. J. Biol. Chem. 2001;276:1424–1433. doi: 10.1074/jbc.M007528200. [DOI] [PubMed] [Google Scholar]
- 3.Blakey D., Leech A., Thomas G. H., Coutts G., Findlay K., Merrick M. Purification of the Escherichia coli ammonium transporter AmtB reveals a trimeric stoichiometry. Biochem. J. 2002;364:527–535. doi: 10.1042/BJ20011761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conroy M. J., Jamieson S. J., Blakey D., Kaufmann T., Engel A., Fotiadis D., Merrick M., Bullough P. A. Electron and atomic force microscopy of the trimeric ammonium transporter AmtB. EMBO Rep. 2004;5:1153–1158. doi: 10.1038/sj.embor.7400296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coutts G., Thomas G., Blakey D., Merrick M. Membrane sequestration of the signal transduction protein GlnK by the ammonium transporter AmtB. EMBO J. 2002;21:1–10. doi: 10.1093/emboj/21.4.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Javelle A., Severi E., Thornton J., Merrick M. Ammonium sensing in E. coli: the role of the ammonium transporter AmtB and AmtB–GlnK complex formation. J. Biol. Chem. 2004;279:8530–8538. doi: 10.1074/jbc.M312399200. [DOI] [PubMed] [Google Scholar]
- 7.Thomas G. H., Mullins J. G., Merrick M. Membrane topology of the Mep/Amt family of ammonium transporters. Mol. Microbiol. 2000;37:331–344. doi: 10.1046/j.1365-2958.2000.01994.x. [DOI] [PubMed] [Google Scholar]
- 8.Kleiner D. Bacterial ammonium transport. FEMS Microbiol. Rev. 1985;32:87–100. [Google Scholar]
- 9.Meier-Wagner J., Nolden L., Jakoby M., Siewe R., Krämer R., Burkovski A. Multiplicity of ammonium uptake systems in Corynebacterium glutamicum: role of Amt and AmtB. Microbiology. 2001;147:135–143. doi: 10.1099/00221287-147-1-135. [DOI] [PubMed] [Google Scholar]
- 10.Ludewig U., von Wirén N., Frommer W. B. Uniport of NH4+ by the root hair plasma membrane ammonium transporter LeAMT1;1. J. Biol. Chem. 2002;277:13548–13555. doi: 10.1074/jbc.M200739200. [DOI] [PubMed] [Google Scholar]
- 11.Ludewig U. Electroneutral ammonium transport by basolateral rhesus B glycoprotein. J. Physiol. 2004;559:751–759. doi: 10.1113/jphysiol.2004.067728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakhoul N. L., Dejong H., Abdulnour-Nakhoul S. M., Boulpaep E. L., Hering-Smith K., Hamm L. L. Characteristics of renal RhBG as an NH4+ transporter. Am. J. Physiol. Renal Physiol. 2005;288:F170–F181. doi: 10.1152/ajprenal.00419.2003. [DOI] [PubMed] [Google Scholar]
- 13.Soupene E., He L., Yan D., Kustu S. Ammonia acquisition in enteric bacteria: physiological role of the ammonium/methylammonium transport B (AmtB) protein. Proc. Natl. Acad. Sci. U.S.A. 1998;95:7030–7034. doi: 10.1073/pnas.95.12.7030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soupene E., Ramirez R. M., Kustu S. Evidence that fungal MEP proteins mediate diffusion of the uncharged species NH3 across the cytoplasmic membrane. Mol. Cell. Biol. 2001;21:5733–5741. doi: 10.1128/MCB.21.17.5733-5741.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khademi S., O'Connell J., III, Remis J., Robles-Colmenares Y., Miercke L. J., Stroud R. M. Mechanism of ammonia transport by Amt/MEP/Rh: structure of AmtB at 1.35 Å. Science. 2004;305:1587–1594. doi: 10.1126/science.1101952. [DOI] [PubMed] [Google Scholar]
- 16.Zheng L., Kostrewa D., Bernèche S., Winkler F. K., Li X.-D. The mechanism of ammonia transport based on the crystal structure of AmtB of E. coli. Proc. Natl. Acad. Sci. U.S.A. 2004;101:17090–17095. doi: 10.1073/pnas.0406475101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ripoche P., Bertrand O., Gane P., Birkenmeier C., Colin Y., Cartron J.-P. Human Rhesus-associated glycoprotein mediates facilitated transport of NH3 into red blood cells. Proc. Natl. Acad. Sci. U.S.A. 2004;101:17222–17227. doi: 10.1073/pnas.0403704101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J. Bacteriol. 1974;119:736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jayakumar A., Barnes E. M., Jr A filtration method for measuring cellular uptake of [14C]methylamine and other highly permeant solutes. Anal. Biochem. 1983;135:475–478. doi: 10.1016/0003-2697(83)90715-7. [DOI] [PubMed] [Google Scholar]
- 20.Marini A.-M., Soussi-Boudekou S., Vissers S., Andre B. A family of ammonium transporters in Saccharomyces cerevisae. Mol. Cell. Biol. 1997;17:4282–4293. doi: 10.1128/mcb.17.8.4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colanduoni J., Nissan R., Villafranca J. J. Studies of the mechanism of glutamine synthetase utilizing pH-dependent behavior in catalysis and binding. J. Biol. Chem. 1987;262:3037–3043. [PubMed] [Google Scholar]
- 22.Pahel G., Zelenetz A. D., Tyler B. M. gltB gene and regulation of nitrogen metabolism by glutamine synthetase in Escherichia coli. J. Bacteriol. 1978;133:139–148. doi: 10.1128/jb.133.1.139-148.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shrake A., Fisher M. T., McFarland P. J., Ginsburg A. Partial unfolding of dodecameric glutamine synthetase from Escherichia coli: temperature-induced, reversible transitions of two domains. Biochemistry. 1989;28:6281–6294. doi: 10.1021/bi00441a021. [DOI] [PubMed] [Google Scholar]
- 24.Agre P., King L. S., Yasui M., Guggino W. B., Ottersen O. P., Fujiyoshi Y., Engel A., Nielsen S. Aquaporin water channels – from atomic structure to clinical medicine. J. Physiol. 2002;542:3–16. doi: 10.1113/jphysiol.2002.020818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu G., Hinch B., Davatol-Hag H., Lu Y., Powers M., Beavis A. D. Temperature dependence of the mitochondrial inner membrane anion channel: the relationship between temperature and inhibition by protons. J Biol. Chem. 1996;271:19717–19723. doi: 10.1074/jbc.271.33.19717. [DOI] [PubMed] [Google Scholar]
- 26.Labotka R. J., Lundberg P., Kuchel P. W. Ammonia permeability of erythrocyte membrane studied by 14N and 15N saturation transfer NMR spectroscopy. Am. J. Physiol. 1995;268:C686–C699. doi: 10.1152/ajpcell.1995.268.3.C686. [DOI] [PubMed] [Google Scholar]
- 27.Possot O. M., Letellier L., Pugsley A. P. Energy requirement for pullulanase secretion by the main terminal branch of the general secretory pathway. Mol. Microbiol. 1997;24:457–464. doi: 10.1046/j.1365-2958.1997.3451726.x. [DOI] [PubMed] [Google Scholar]
- 28.Ninnemann O., Jauniaux J.-C., Frommer W. B. Identification of a high affinity NH4+ transporter from plants. EMBO J. 1994;13:3464–3471. doi: 10.1002/j.1460-2075.1994.tb06652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siewe R. M., Weil B., Burkovski A., Eikmanns B. J., Eikmanns M., Krämer R. Functional and genetic characterisation of the (methyl)ammonium uptake carrier of Corynebacterium glutamicum. J. Biol. Chem. 1996;271:5398–5403. doi: 10.1074/jbc.271.10.5398. [DOI] [PubMed] [Google Scholar]
- 30.Alibhai M., Villafranca J. J. Kinetic and mutagenic studies of the role of the active site residues Asp-50 and Glu-327 of Escherichia coli glutamine synthetase. Biochemistry. 1994;33:682–686. doi: 10.1021/bi00169a008. [DOI] [PubMed] [Google Scholar]
- 31.Barnes E. M., Jr, Zimniak P., Jayakumar A. Role of glutamine synthetase in the uptake and metabolism of methylammonium by Azotobacter vinelandii. J. Bacteriol. 1983;156:752–757. doi: 10.1128/jb.156.2.752-757.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kleinschmidt J. A., Kleiner D. The glutamine synthetase from Azotobacter vinelandii: purification, characterisation, regulation and localisation. Eur. J. Biochem. 1978;89:51–60. doi: 10.1111/j.1432-1033.1978.tb20895.x. [DOI] [PubMed] [Google Scholar]
- 33.Barnes E. M., Jr, Zimniak P. Transport of ammonium and methylammonium ions by Azotobacter vinelandii. J. Bacteriol. 1981;146:512–516. doi: 10.1128/jb.146.2.512-516.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maurel C., Reizer J., Schroeder J. I., Chrispeels M. J., Saier M. H., Jr Functional characterization of the Escherichia coli glycerol facilitator, GlpF, in Xenopus oocytes. J. Biol. Chem. 1994;269:11869–11872. [PubMed] [Google Scholar]
- 35.Voegele R. T., Sweet G. D., Boos W. Glycerol kinase of Escherichia coli is activated by interaction with the glycerol facilitator. J. Bacteriol. 1993;175:1087–1094. doi: 10.1128/jb.175.4.1087-1094.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gutknecht J., Walter A. Histamine, theophylline and tryptamine transport through lipid bilayer membranes. Biochim. Biophys. Acta. 1981;649:149–154. doi: 10.1016/0005-2736(81)90401-6. [DOI] [PubMed] [Google Scholar]
- 37.Barry P. H., Diamond J. M. Effects of unstirred layers on membrane phenomena. Physiol. Rev. 1984;64:763–872. doi: 10.1152/physrev.1984.64.3.763. [DOI] [PubMed] [Google Scholar]
- 38.Accardi A., Miller C. Secondary active transport mediated by a prokaryotic homologue of ClC Cl− channels. Nature (London) 2004;427:803–807. doi: 10.1038/nature02314. [DOI] [PubMed] [Google Scholar]
- 39.Booth I. R., Edwards M. D., Miller S. Bacterial ion channels. Biochemistry. 2003;42:10045–10053. doi: 10.1021/bi034953w. [DOI] [PubMed] [Google Scholar]
- 40.MacNeil D. General method, using Mu–Mud1 dilysogens, to determine the direction of transcription of and generate deletions in the glnA region of Escherichia coli. J. Bacteriol. 1981;146:260–268. doi: 10.1128/jb.146.1.260-268.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jayakumar A., Schulman I., Macneil D., Barnes E. M., Jr Role of the Escherichia coli glnALG operon in regulation of ammonium transport. J. Bacteriol. 1986;166:281–284. doi: 10.1128/jb.166.1.281-284.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mumberg D., Muller R., Funk M. Regulatable promoters of Saccharomyces cerevisiae: comparison of transcriptional activity and their use for heterologous expression. Nucleic Acids Res. 1994;22:5767–5768. doi: 10.1093/nar/22.25.5767. [DOI] [PMC free article] [PubMed] [Google Scholar]