Abstract
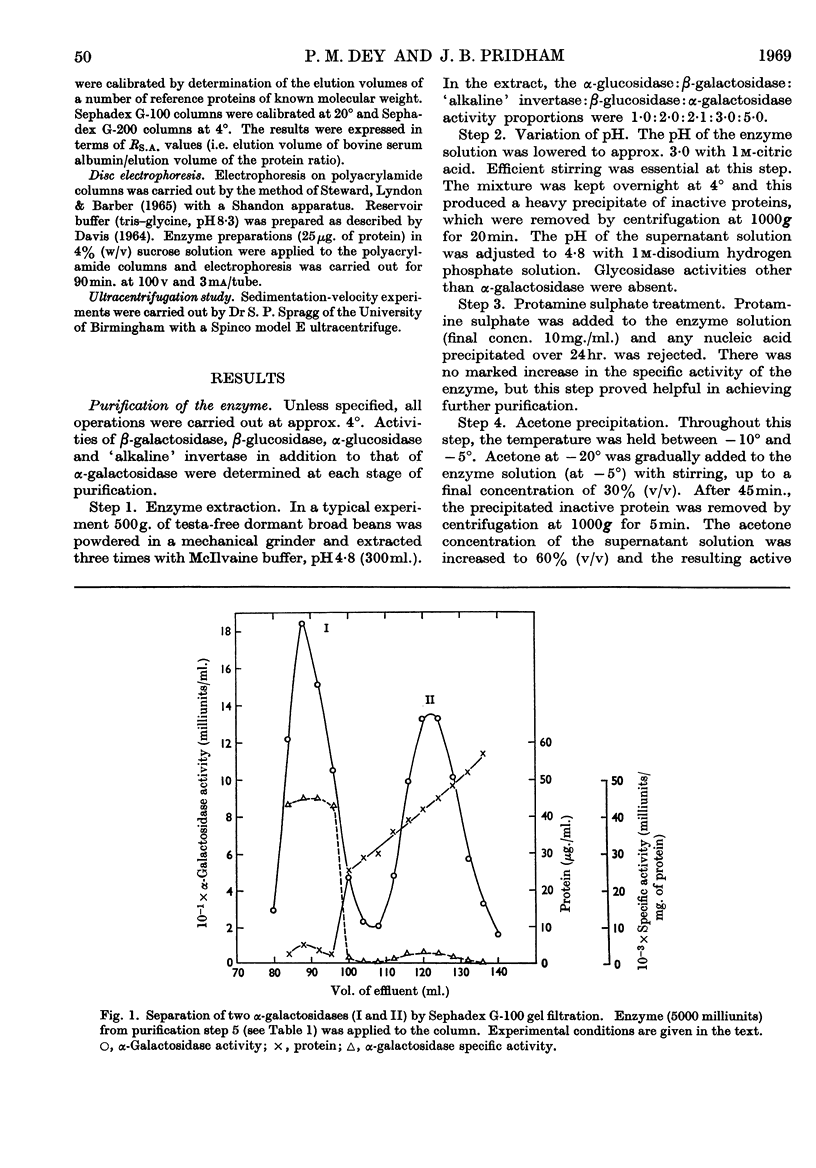
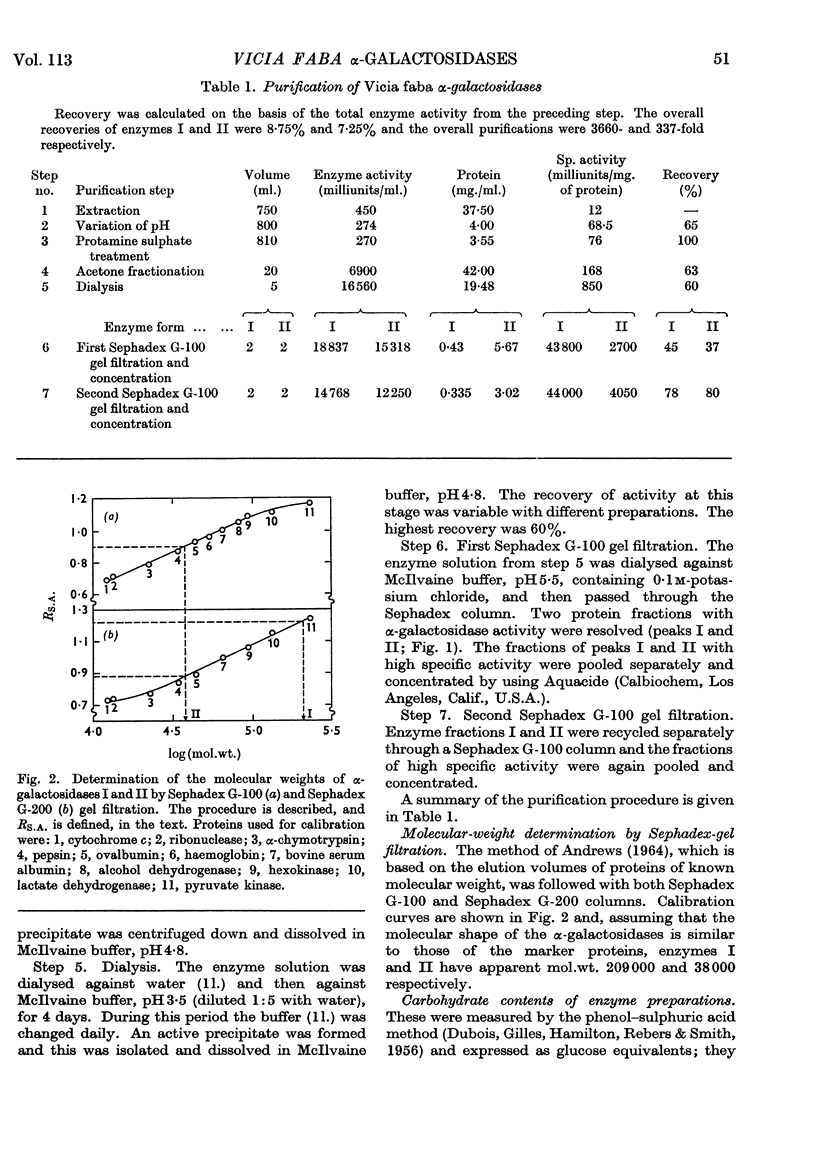
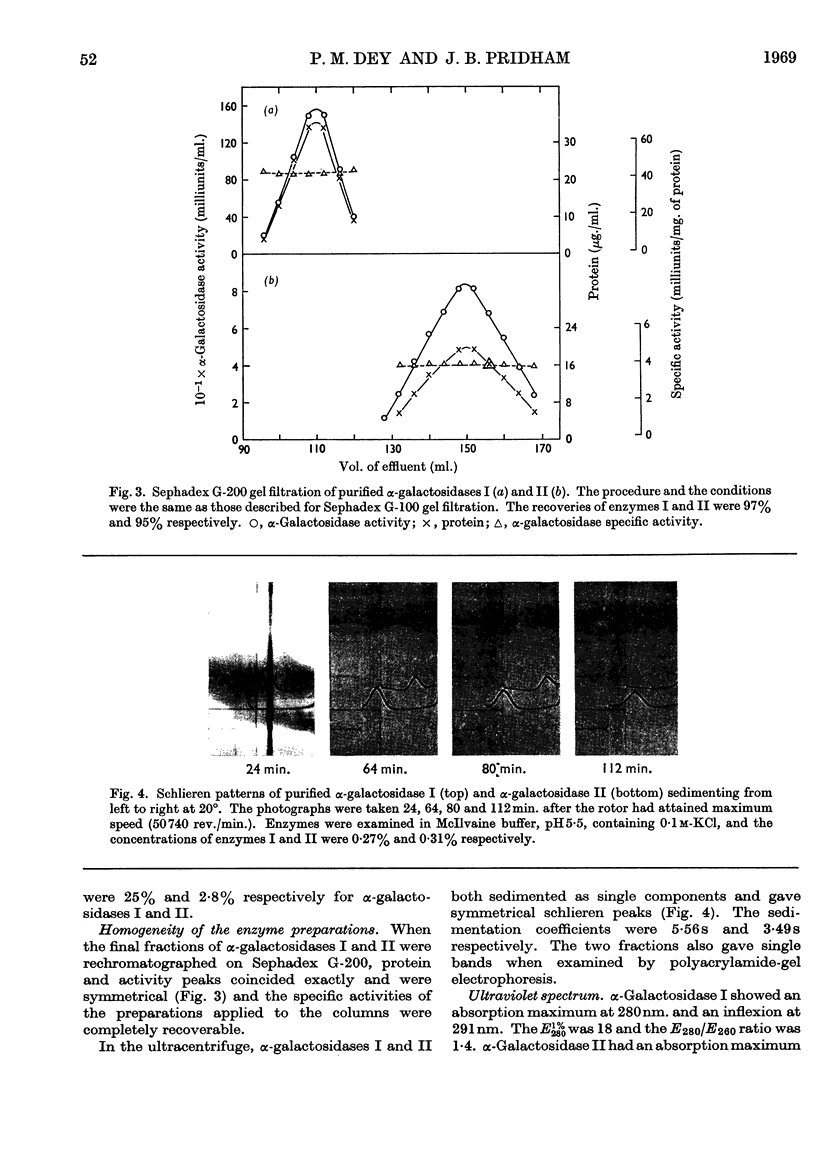
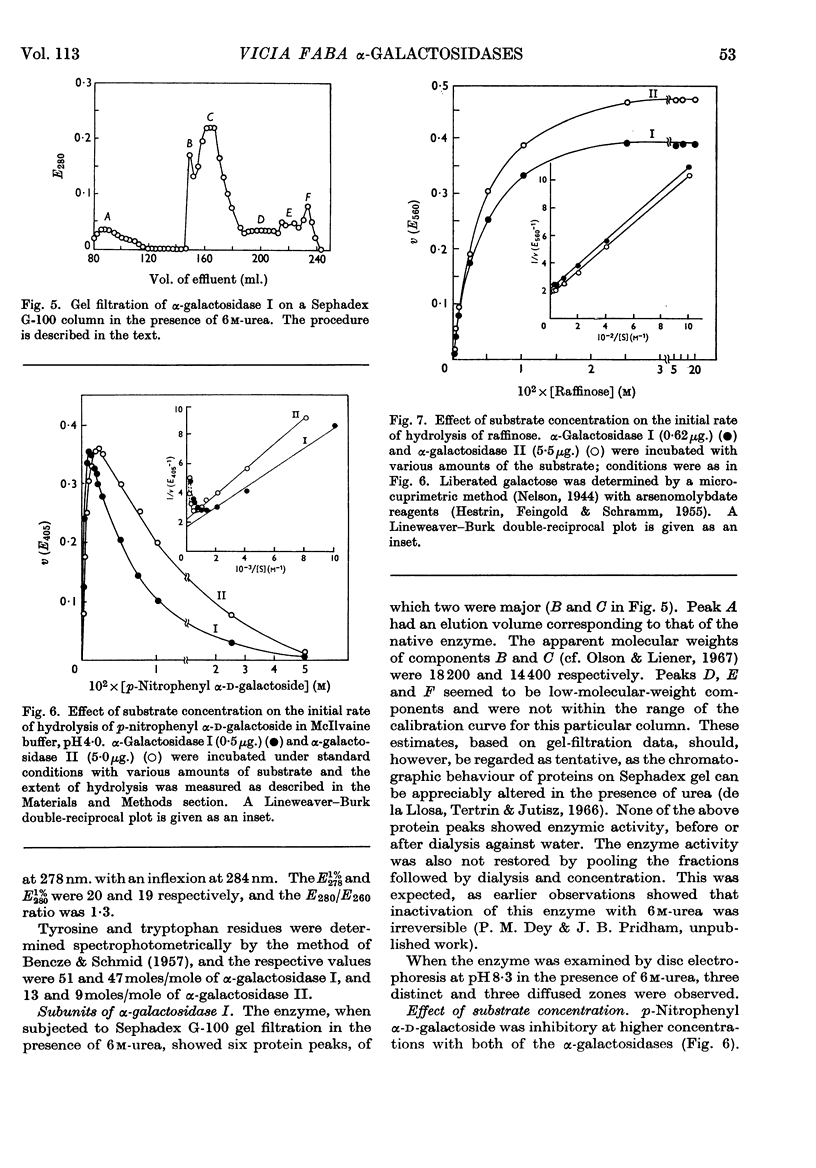
Two forms of α-galactosidase, I and II, exist in Vicia faba seeds and these have been purified 3660- and 337-fold respectively. They behaved as homogeneous preparations when examined by ultracentrifugation, disc electrophoresis and gel filtration. The apparent molecular weights of enzymes I and II, as determined by gel filtration, were 209000 and 38000 respectively. The carbohydrate contents of enzymes I and II were 25% and 2·8% respectively, and the enzymes differed in their aromatic amino acid compositions. Enzyme I was split into six inactive subunits in the presence of 6m-urea. α-Galactosidases I and II showed different pH optima and Km and Vmax. values with p-nitrophenyl α-d-galactoside and raffinose as substrates, and also differed in their thermal stabilities.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AHMED M. U., COOK F. S. SEPARATION OF AN ALPHA-GALACTOSIDASE FROM WATERMELON SEEDS. Can J Biochem. 1964 May;42:605–612. doi: 10.1139/o64-073. [DOI] [PubMed] [Google Scholar]
- Agrawal K. M., Bahl O. P. Glycosidases of Phaseolus vulgaris. II. Isolation and general properties. J Biol Chem. 1968 Jan 10;243(1):103–111. [PubMed] [Google Scholar]
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MALHOTRA O. P., DEY P. M. STUDIES IN GALACTOSE TRANSFERRING ENZYMES; PRELIMINARY COMMUNICATION. Biochem Z. 1964 Nov 6;340:565–566. [PubMed] [Google Scholar]
- Malhotra O. P., Dey P. M. Purification and physical properties of sweet-almond alpha-galactosidase. Biochem J. 1967 May;103(2):508–513. doi: 10.1042/bj1030508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson M. O., Liener I. E. The association and dissociation of concanavalin A, the phytohemagglutinin of the jack bean. Biochemistry. 1967 Dec;6(12):3801–3808. doi: 10.1021/bi00864a025. [DOI] [PubMed] [Google Scholar]
- PAZUR J. H., SHADAKSHARASWAMY M., MEIDELL G. E. The metabolism of oligosaccharides in germinating soybeans, Glycine max. Arch Biochem Biophys. 1962 Oct;99:78–85. doi: 10.1016/0003-9861(62)90246-1. [DOI] [PubMed] [Google Scholar]
- PETEK F., DONG T. [Separation and study of 2 alpha-galactosidases from coffee beans]. Enzymologia. 1961 Jun 30;23:133–142. [PubMed] [Google Scholar]
- PRIDHAM J. B. Occurrence and metabolism of oligosaccharides in the broad bean (Vicia faba). Nature. 1958 Dec 13;182(4650):1687–1688. doi: 10.1038/1821687a0. [DOI] [PubMed] [Google Scholar]
- STEWARD F. C., LYNDON R. F., BARBER J. T. ACRYLAMIDE GEL ELECTROPHORESIS OF SOLUBLE PLANT PROTEINS: A STUDY ON PEA SEEDLINGS IN RELATION TO DEVELOPMENT. Am J Bot. 1965 Feb;52:155–164. [PubMed] [Google Scholar]



