Abstract
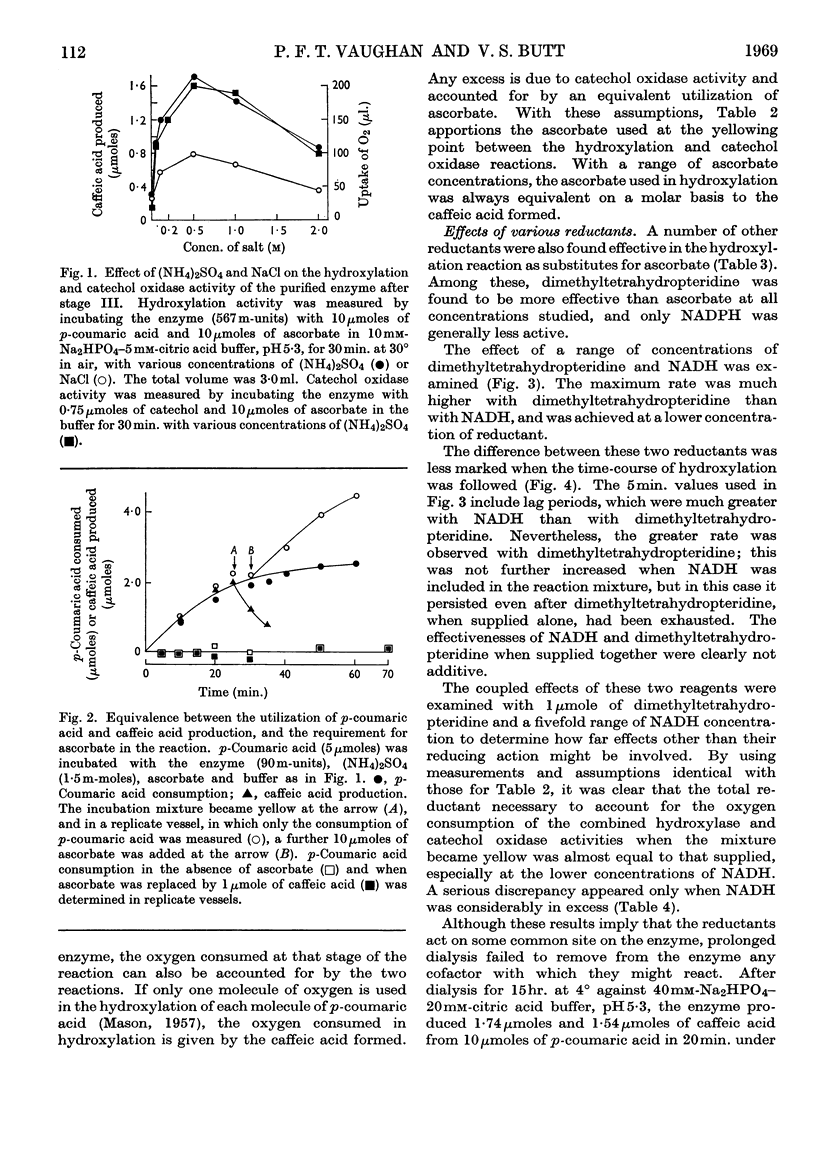
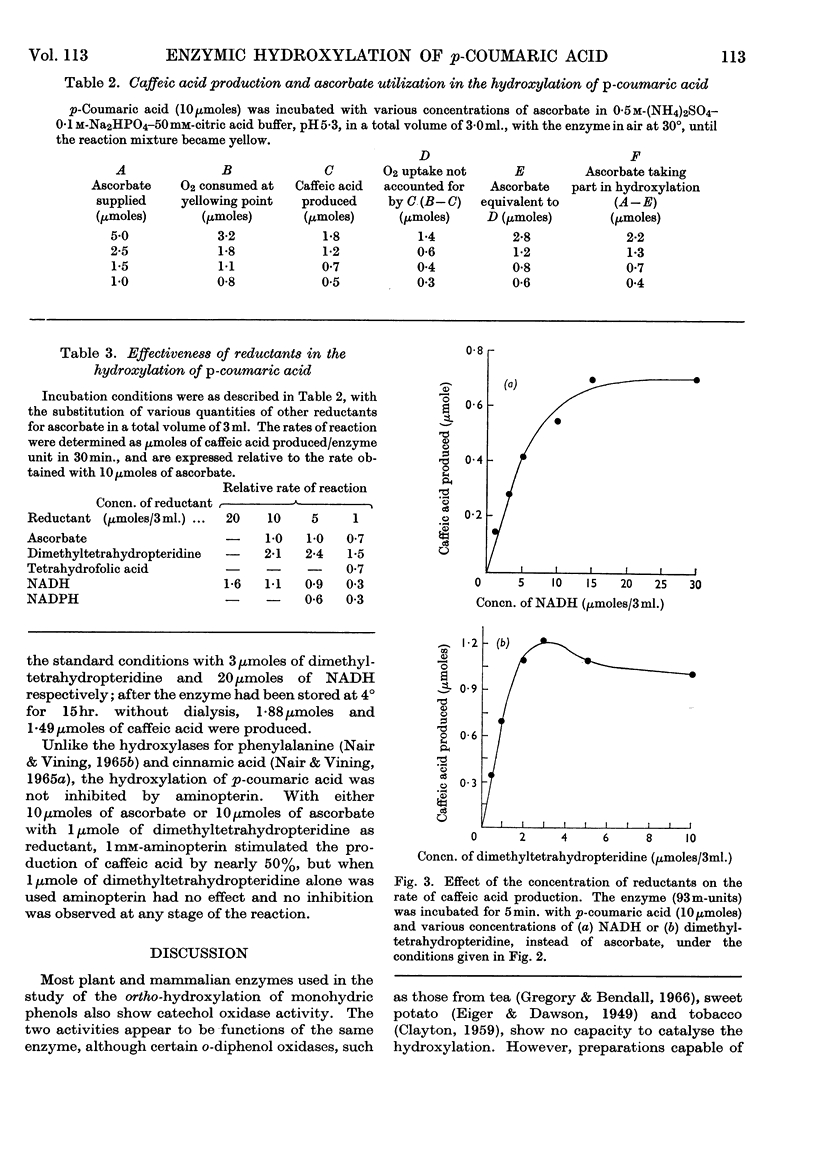
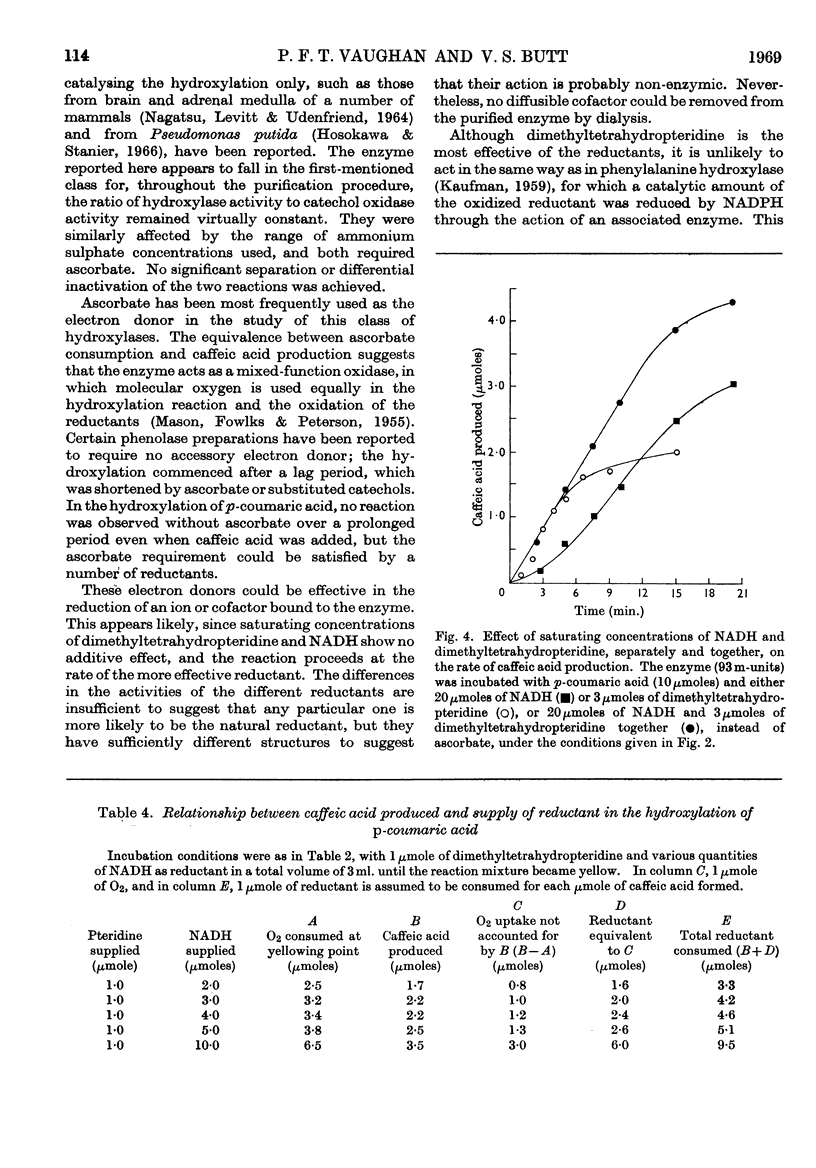
1. An enzyme from the leaves of spinach beet (Beta vulgaris L.) that catalyses the hydroxylation of p-coumaric acid to caffeic acid in the presence of ascorbate has been purified about 1000-fold on a protein basis. 2. It is activated by high concentrations of ammonium sulphate and sodium chloride. 3. The preparation shows both hydroxylase and catechol oxidase activities, in a constant ratio throughout the purification procedure; they are similarly activated by salts. 4. Ascorbate acts as a reductant in quantities equivalent to the caffeic acid produced by hydroxylation. 5. Ascorbate can be replaced by tetrahydrofolic acid, NADH, NADPH or 2-amino-4-hydroxy-6,7-dimethyl-5,6,7,8-tetrahydropteridine, but not by caffeic acid. Among these, the pteridine is the most effective, but the reaction is not inhibited by aminopterin. In experiments with saturating concentrations of NADH and the pteridine, these reductants compete in the reaction and are equivalent on a molar basis. 6. No cofactor has been separated from the enzyme by prolonged dialysis. 7. The relation of the enzyme to other hydroxylases and phenolases is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CLAYTON R. A. Properties of tobacco polyphenol oxidase. Arch Biochem Biophys. 1959 Apr;81(2):404–417. doi: 10.1016/0003-9861(59)90219-x. [DOI] [PubMed] [Google Scholar]
- Gregory R. P., Bendall D. S. The purification and some properties of the polyphenol oxidase from tea (Camellia sinensis L.). Biochem J. 1966 Dec;101(3):569–581. doi: 10.1042/bj1010569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa K., Stanier R. Y. Crystallization and properties of p-hydroxybenzoate hydroxylase from Pseudomonas putida. J Biol Chem. 1966 May 25;241(10):2453–2460. [PubMed] [Google Scholar]
- KAUFMAN S. Studies on the mechanism of the enzymatic conversion of phenylalanine to tyrosine. J Biol Chem. 1959 Oct;234:2677–2682. [PubMed] [Google Scholar]
- Lugg J. W. On the use of mercuric salts and nitrous acid in the colorimetric determination of tyrosine and tryptophan present in solution. Biochem J. 1937 Aug;31(8):1422–1433. doi: 10.1042/bj0311422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASON H. S. Mechanisms of oxygen metabolism. Adv Enzymol Relat Subj Biochem. 1957;19:79–233. doi: 10.1002/9780470122648.ch2. [DOI] [PubMed] [Google Scholar]
- NAGATSU T., LEVITT M., UDENFRIEND S. TYROSINE HYDROXYLASE. THE INITIAL STEP IN NOREPINEPHRINE BIOSYNTHESIS. J Biol Chem. 1964 Sep;239:2910–2917. [PubMed] [Google Scholar]
- Patil S. S., Zucker M. Potato phenolases. Purification and properties. J Biol Chem. 1965 Oct;240(10):3938–3943. [PubMed] [Google Scholar]
- Pomerantz S. H. The tyrosine hydroxylase activity of mammalian tyrosinase. J Biol Chem. 1966 Jan 10;241(1):161–168. [PubMed] [Google Scholar]
- Vaughan P. F., Butt V. S. Electron-donor requirements in the hydroxylation of p-coumaric acid. Biochem J. 1967 Sep;104(3):65P–66P. [PMC free article] [PubMed] [Google Scholar]


