Abstract
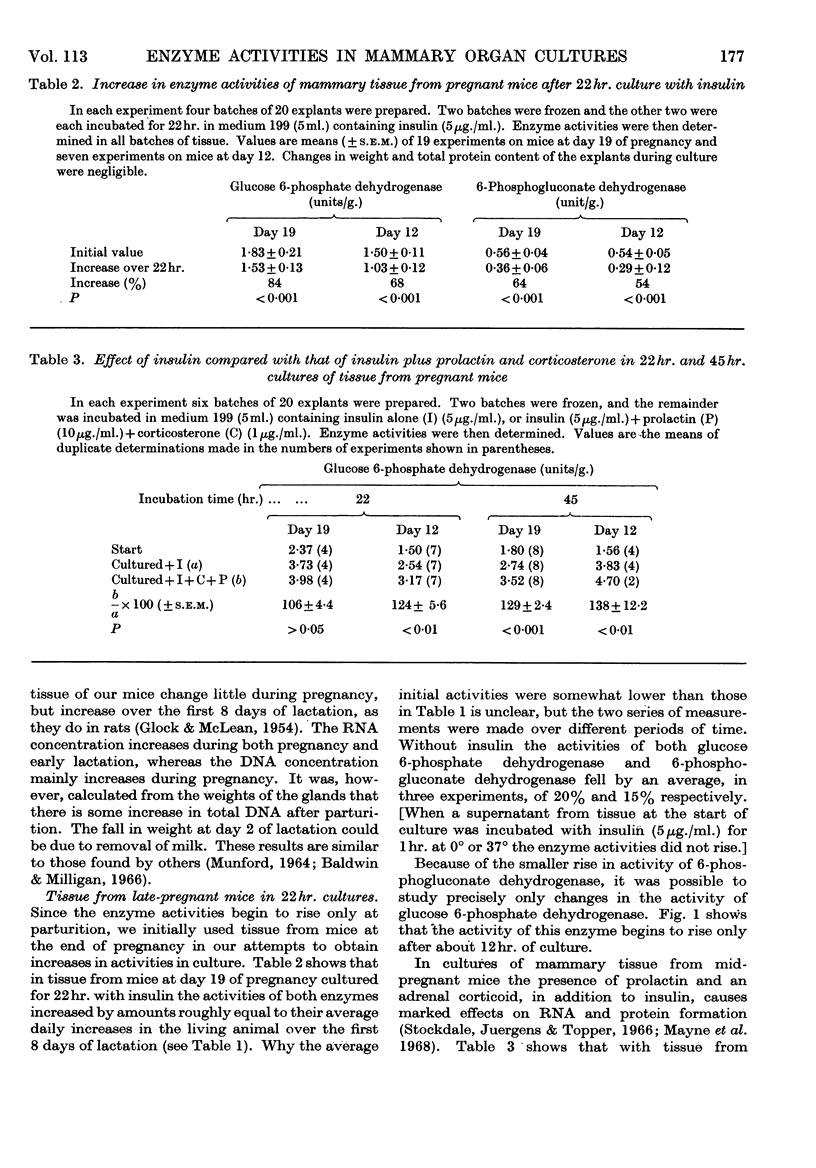
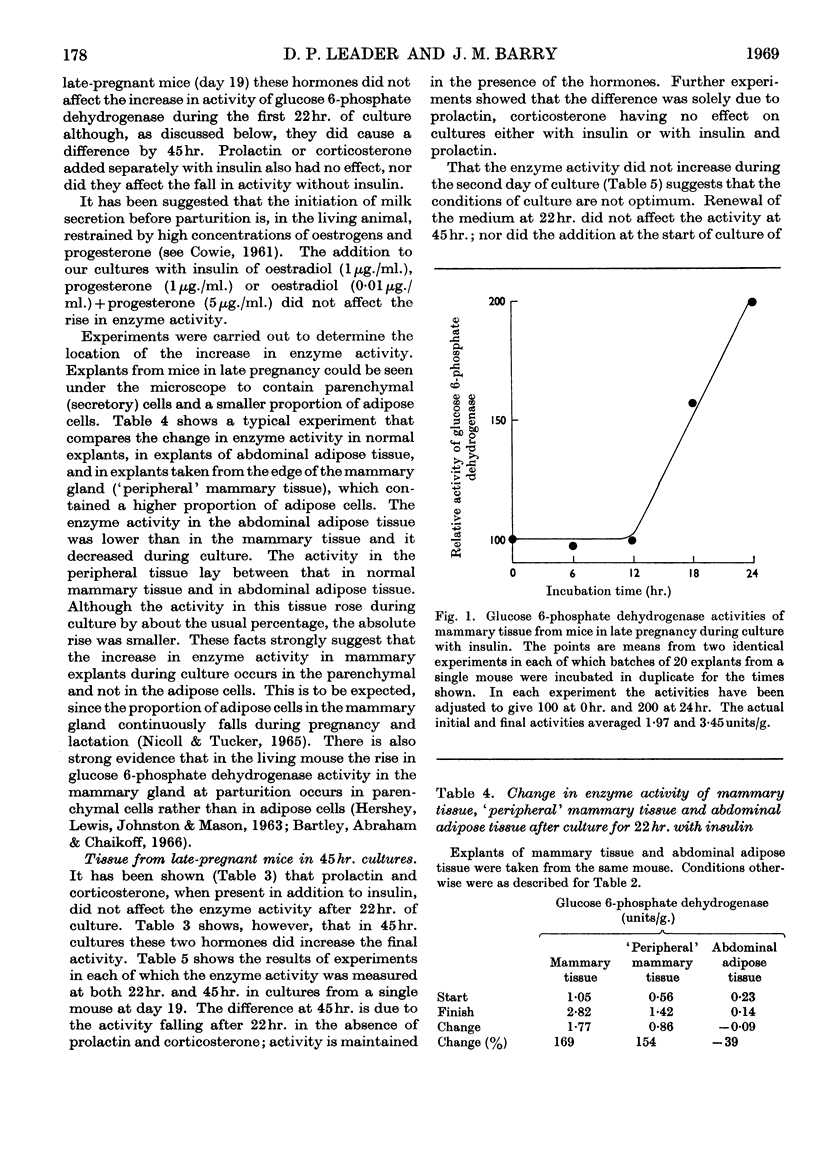
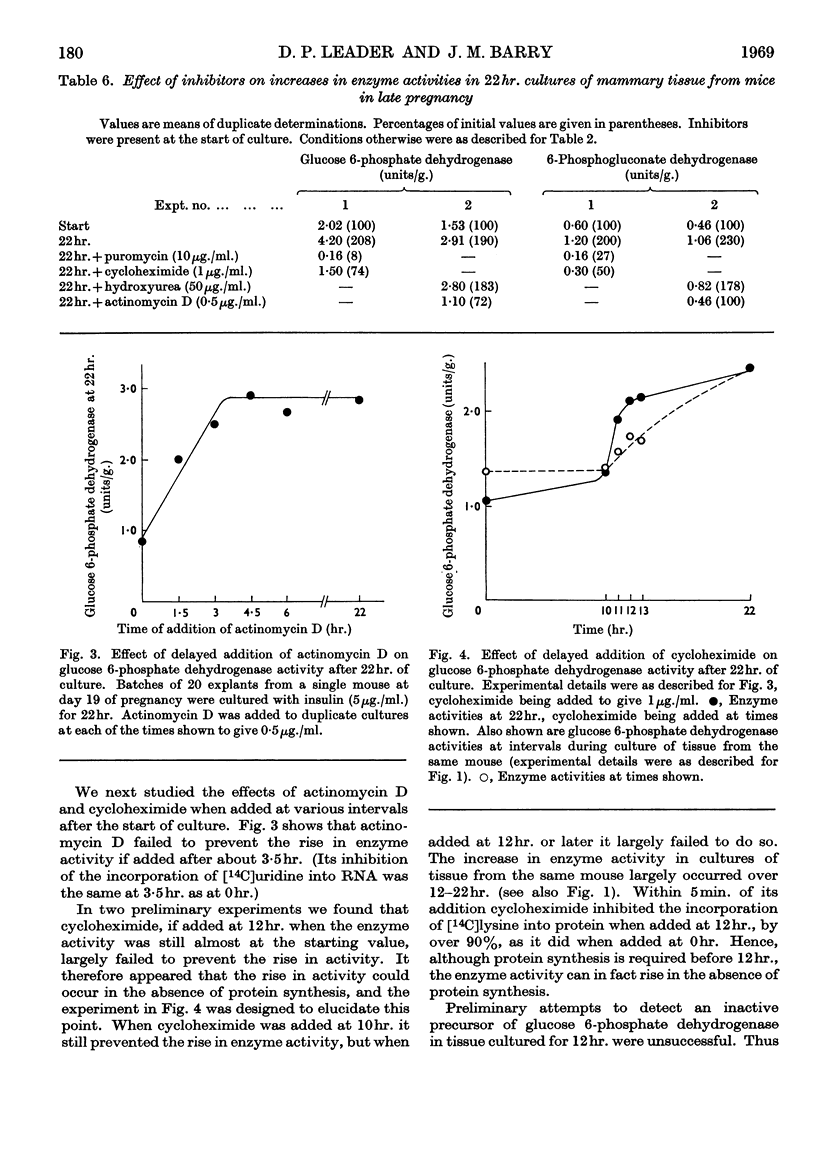
1. In organ cultures of mammary tissue from C3H mice we observed increases in the activity of glucose 6-phosphate dehydrogenase similar to that occurring at parturition. 2. In 22hr. cultures of tissue from late-pregnant mice insulin was required for the increases, but the further addition of prolactin, corticosterone and certain other hormones had no effect. The rise in activity occurred over the second half of the culture period. 3. Results from culture of adipose tissue, and mammary tissue rich in adipose tissue, strongly suggest that the rise in activity occurs in mammary parenchymal rather than adipose cells. 4. In 45hr. cultures prolactin prevented a fall in enzyme activity between 22hr. and 45hr. If the medium contained serum the activity at 22hr. was unaffected, but it continued to rise up to 45hr., and prolactin then had no effect. 5. The enzyme also increased in activity in cultures of mammary tissue from mid-pregnant mice. Insulin was again required, the activity was higher at 45hr. than at 24hr. and prolactin increased the activities at both these times. 6. Actinomycin D, cycloheximide and puromycin at low concentration in the media of 22hr. cultures all prevented increases in enzyme activity. Hydroxyurea at a concentration that inhibited the incorporation of [3H]thymidine into DNA by 92% had little effect. 7. Actinomycin D and cycloheximide largely failed to prevent the rise in enzyme activity if added after 3·5hr. and 12hr. respectively. Hence all essential RNA and protein synthesis appears to be finished by 3·5hr. and 12hr., although most of the increase in enzyme activity occurs gradually between 12hr. and 22hr. 8. We suggest that the increases in enzyme activity, both in culture and in the living animal at parturition, are induced by an influx of glucose that is restrained during pregnancy by the growth-hormone-like action of placental lactogen.
Full text
PDF

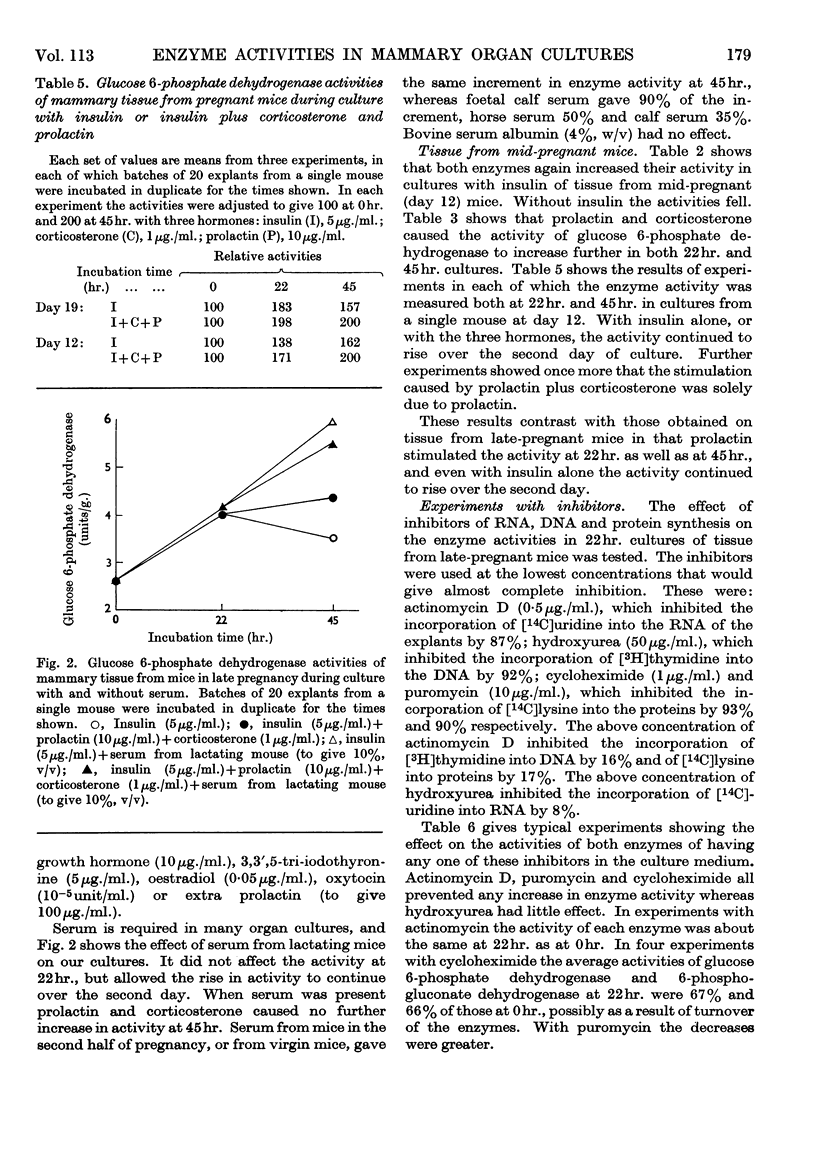


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin R. L., Milligan L. P. Enzymatic changes associated with the initiation and maintenance of lactation in the rat. J Biol Chem. 1966 May 10;241(9):2058–2066. [PubMed] [Google Scholar]
- Bartley J. C., Abraham S., Chaikoff I. L. Activity patterns of several enzymes of liver, adipose tissue, and mammary gland of virgin, pregnant, and lactating mice. Proc Soc Exp Biol Med. 1966 Dec;123(3):670–675. doi: 10.3181/00379727-123-31573. [DOI] [PubMed] [Google Scholar]
- CERRUTI R. A., LYONS W. R. Mammogenic activities of the midgestational mouse placenta. Endocrinology. 1960 Dec;67:884–887. doi: 10.1210/endo-67-6-884. [DOI] [PubMed] [Google Scholar]
- ELIAS J. J. Effect of insulin and cortisol on organ cultures of adult mouse mammary gland. Proc Soc Exp Biol Med. 1959 Jul;101(3):500–502. doi: 10.3181/00379727-101-24995. [DOI] [PubMed] [Google Scholar]
- ELIAS J. J., RIVERA E. Comparison of the responses of normal, precancerous, and neoplastic mouse mammary tissues to hormones in vitro. Cancer Res. 1959 Jun;19(5):505–511. [PubMed] [Google Scholar]
- GLOCK G. E., McLEAN P. Further studies on the properties and assay of glucose 6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase of rat liver. Biochem J. 1953 Oct;55(3):400–408. doi: 10.1042/bj0550400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLOCK G. E., McLEAN P. Levels of enzymes of the direct oxidative pathway of carbohydrate metabolism in mammalian tissues and tumours. Biochem J. 1954 Jan;56(1):171–175. doi: 10.1042/bj0560171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumbach M. M., Kaplan S. L., Sciarra J. J., Burr I. M. Chorionic growth hormone-prolactin (CGP): secretion, disposition, biologic activity in man, and postulated function as the "growth hormone" of the 2d half of pregnancy. Ann N Y Acad Sci. 1968 Feb 5;148(2):501–531. doi: 10.1111/j.1749-6632.1968.tb20372.x. [DOI] [PubMed] [Google Scholar]
- Juergens W. G., Stockdale F. E., Topper Y. J., Elias J. J. Hormone-dependent differentiation of mammary gland in vitro. Proc Natl Acad Sci U S A. 1965 Aug;54(2):629–634. doi: 10.1073/pnas.54.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayne R., Barry J. M. Insulin maintains the level of RNA in mammary tissue culture. Biochim Biophys Acta. 1967 Mar 29;138(1):195–197. doi: 10.1016/0005-2787(67)90602-8. [DOI] [PubMed] [Google Scholar]
- Mayne R., Barry J. M., Rivera E. M. Stimulation by insulin of the formation of ribonucleic acid and protein by mammary tissues in vitro. Biochem J. 1966 Jun;99(3):688–693. doi: 10.1042/bj0990688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayne R., Forsyth I. A., Barry J. M. Stimulation by hormones of RNA and protein formation in organ cultures of the mammary glands of pregnant mice. J Endocrinol. 1968 Jun;41(2):247–253. doi: 10.1677/joe.0.0410247. [DOI] [PubMed] [Google Scholar]
- Nevaldine B. H., Levy H. R. Reversible dissociation and association of mammary glucose-6-phosphate dehydrogenase. Biochem Biophys Res Commun. 1965 Oct 8;21(1):28–33. doi: 10.1016/0006-291x(65)90421-3. [DOI] [PubMed] [Google Scholar]
- Nicoll C. S., Tucker H. A. Estimates of parenchymal, stromal, and lymph node deoxyribonucleic acid in mammary glands of C3H/Crgl-2 mice. Life Sci. 1965 May;4(9):993–1001. doi: 10.1016/0024-3205(65)90203-1. [DOI] [PubMed] [Google Scholar]
- RIVERA E. M., BERN H. A. Influence of insulin on maintenance and secretory stimulation of mouse mammary tissues by hormones in organ-culture. Endocrinology. 1961 Aug;69:340–353. doi: 10.1210/endo-69-2-340. [DOI] [PubMed] [Google Scholar]
- SPELLACY W. N., GOETZ F. C. Plasma insulin in normal late pregnancy. N Engl J Med. 1963 May 2;268:988–991. doi: 10.1056/NEJM196305022681805. [DOI] [PubMed] [Google Scholar]
- Stockdale F. E., Juergens W. G., Topper Y. J. A histological and biochemical study of hormone-dependent differentiation of mammary gland tissue in vitro. Dev Biol. 1966 Apr;13(2):266–281. doi: 10.1016/0012-1606(66)90068-6. [DOI] [PubMed] [Google Scholar]
- Stockdale F. E., Topper Y. J. The role of DNA synthesis and mitosis in hormone-dependent differentiation. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1283–1289. doi: 10.1073/pnas.56.4.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata J. R. The formation and distribution of ribosomes during hormone-induced growth and development. Biochem J. 1967 Jul;104(1):1–16. doi: 10.1042/bj1040001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkington R. W., Brew K., Vanaman T. C., Hill R. L. The hormonal control of lactose synthetase in the developing mouse mammary gland. J Biol Chem. 1968 Jun 25;243(12):3382–3387. [PubMed] [Google Scholar]


