Abstract
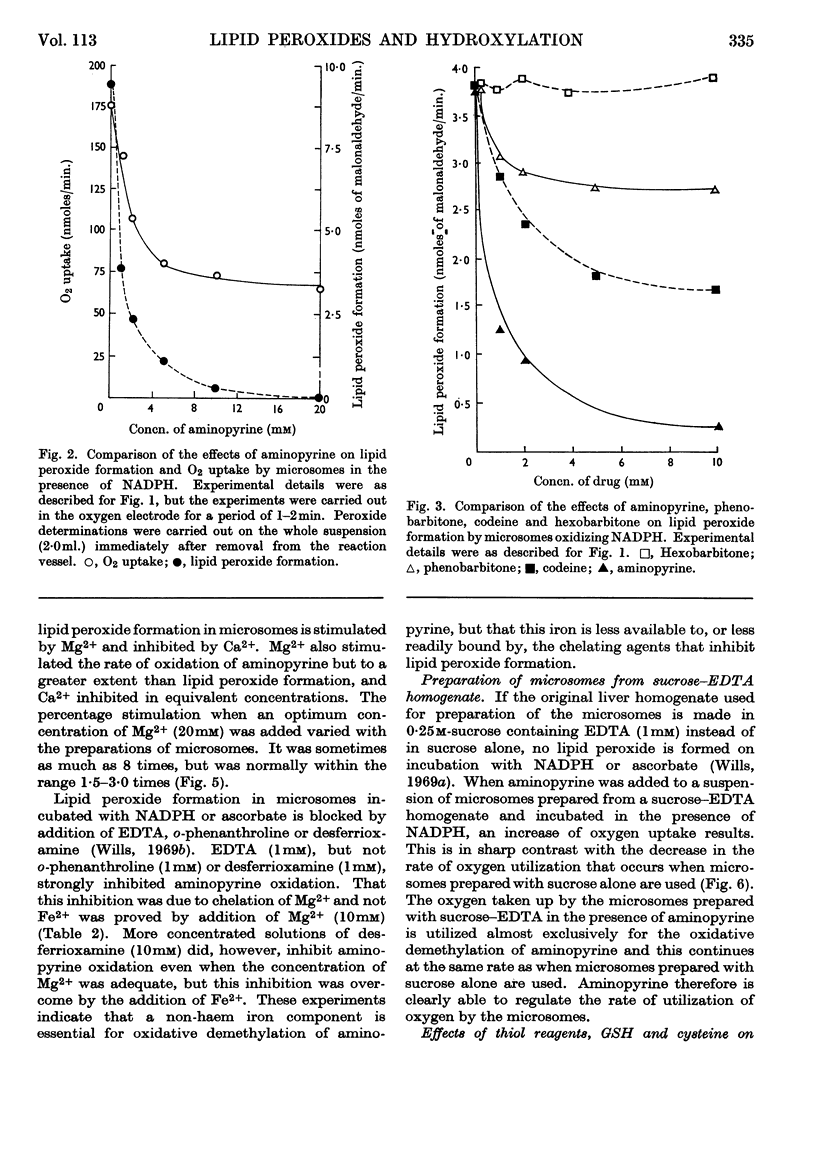
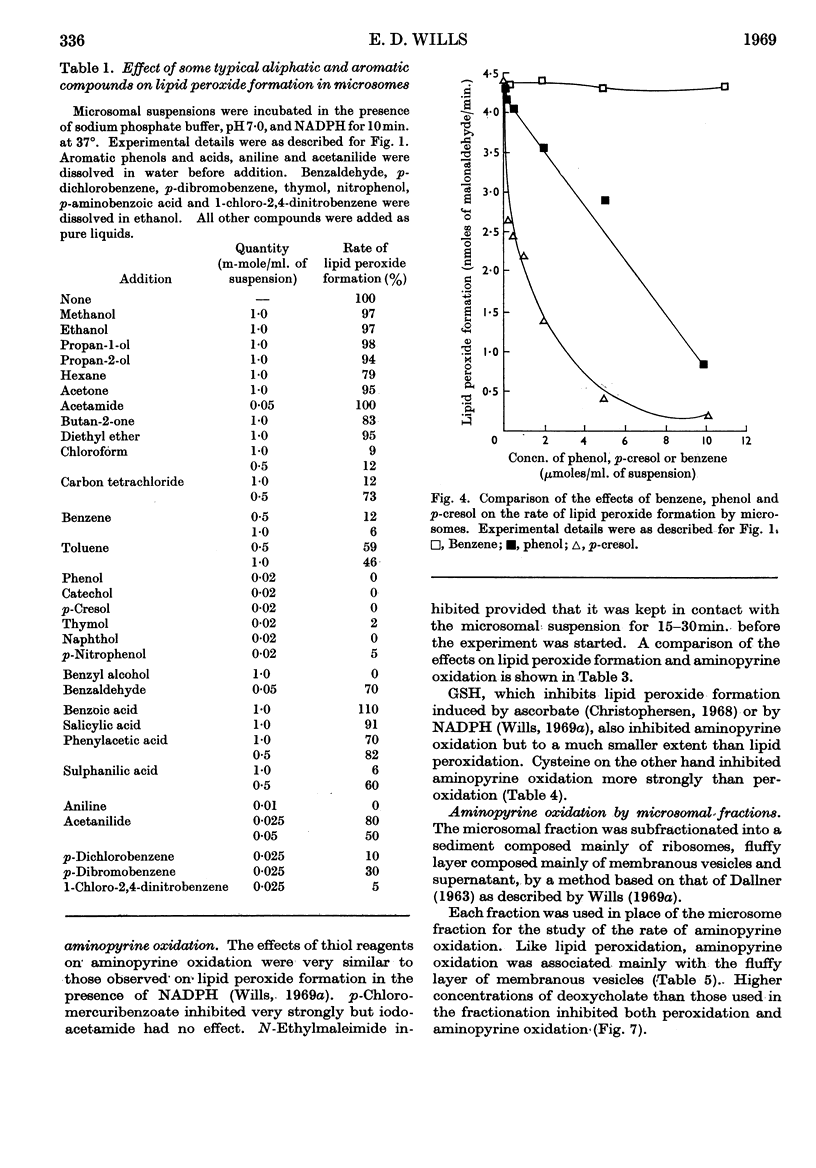
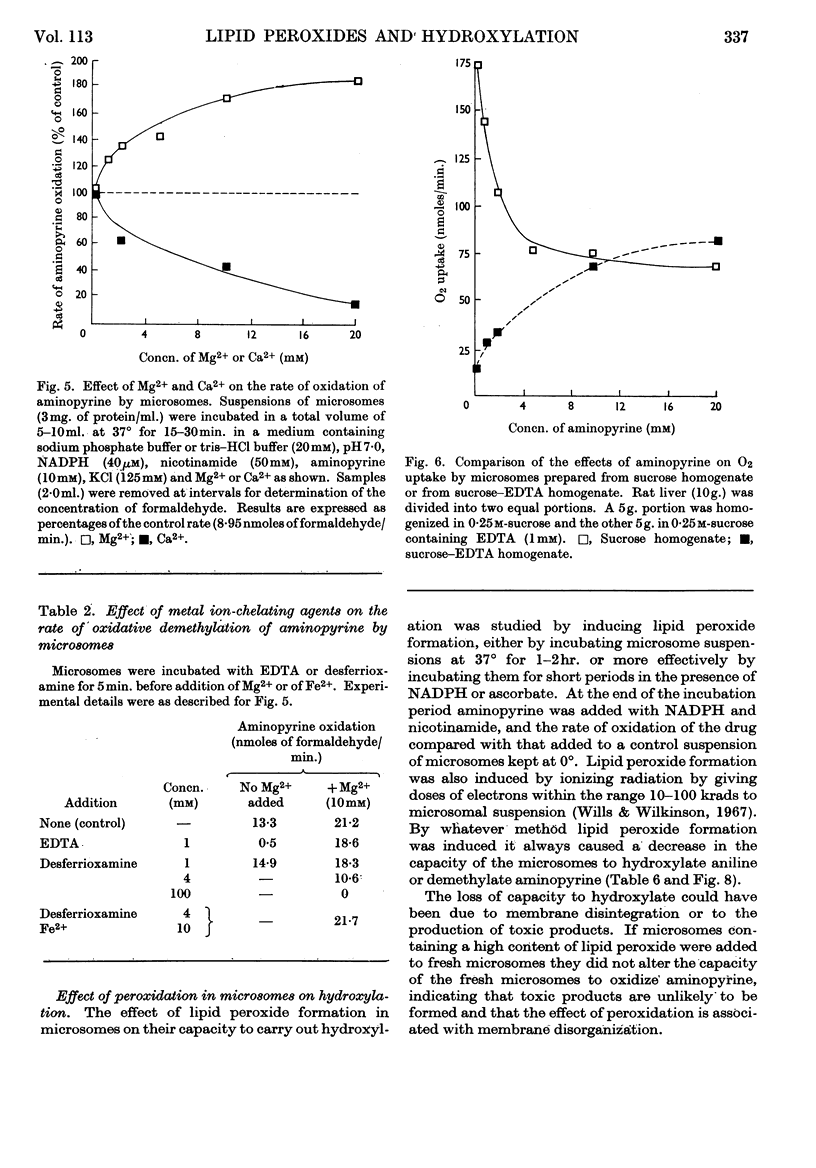
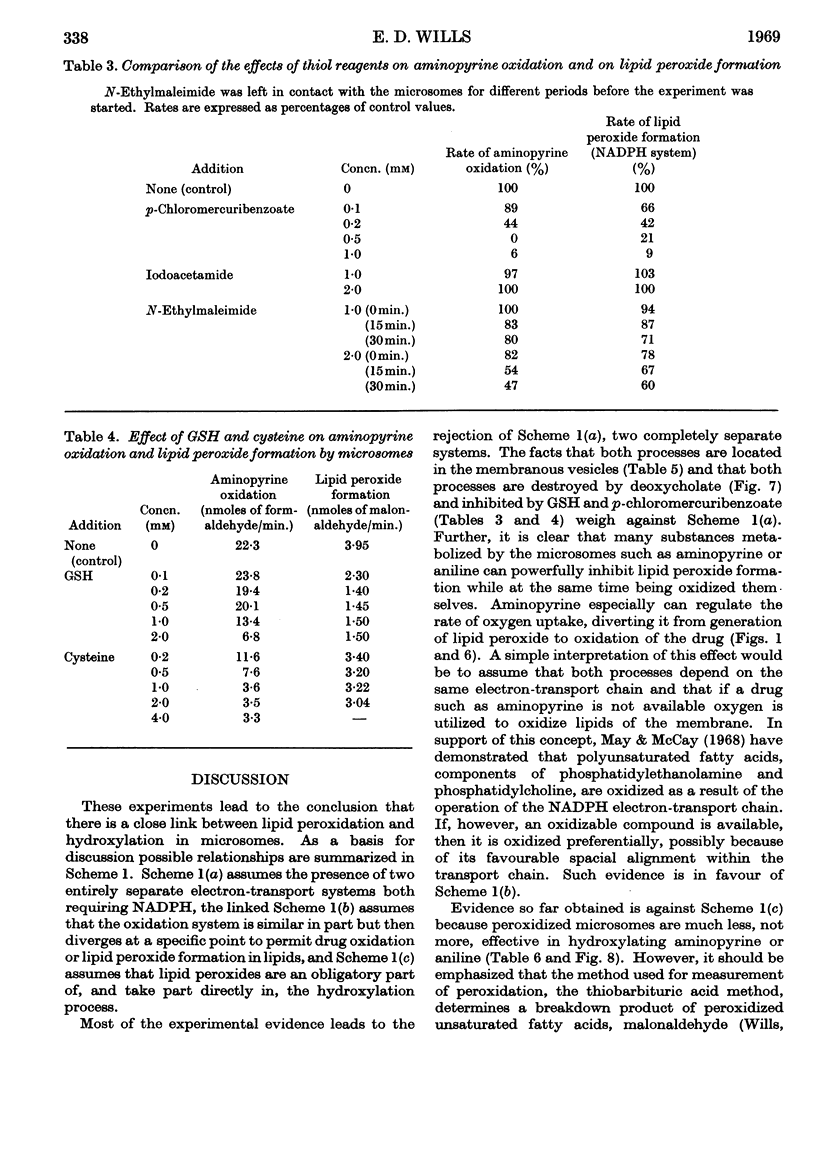
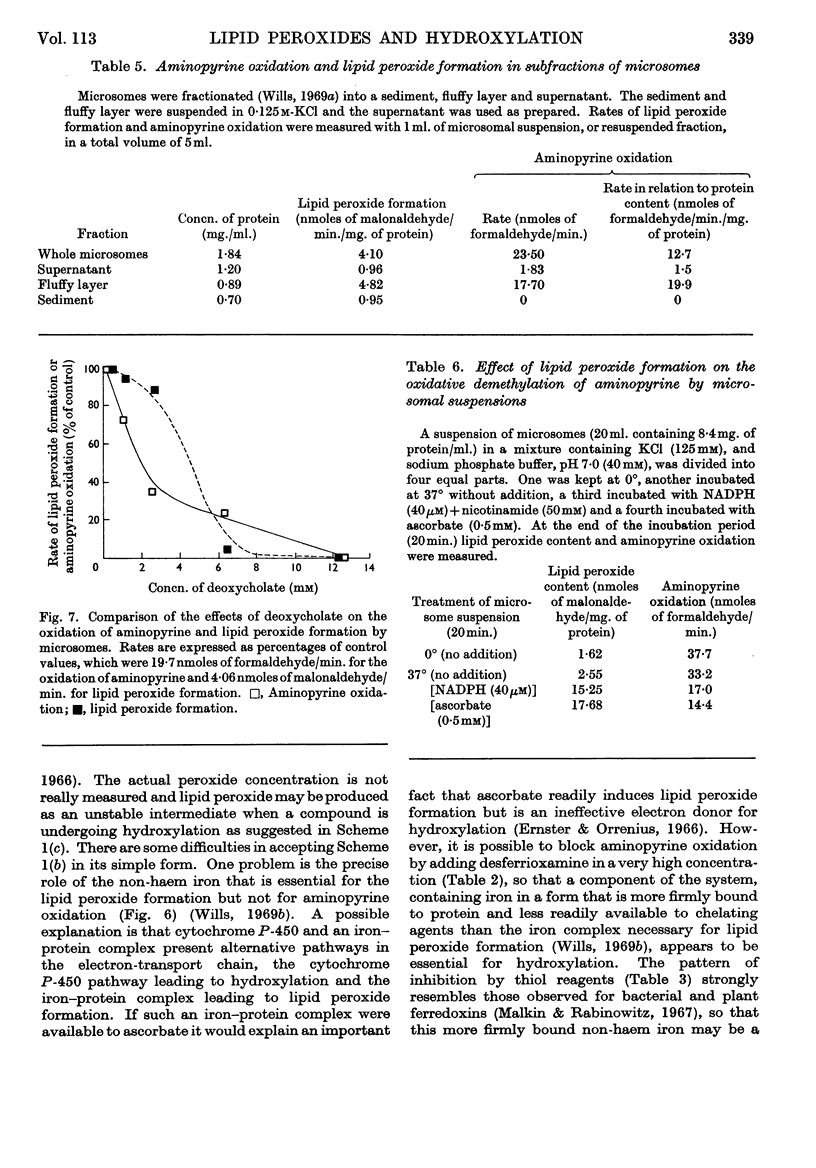
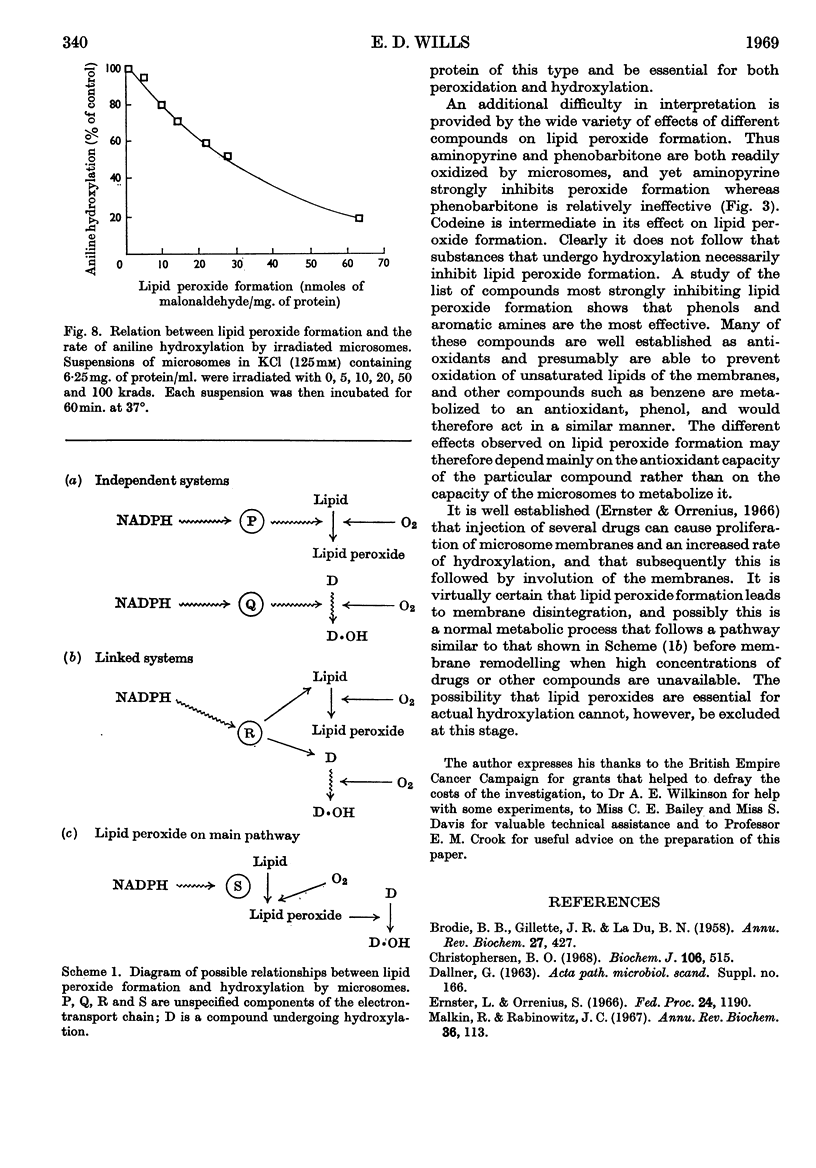
1. Aminopyrine strongly inhibits NADPH-induced lipid peroxide formation in rat liver microsomes, but ascorbate-induced peroxidation is inhibited to a smaller extent. 2. Aminopyrine oxidation is stimulated by Mg2+ but inhibited by Ca2+. Concentrated solutions (10mm) of iron-chelating agents inhibit aminopyrine oxidation, but the more dilute solutions (0·5mm) of chelators that block lipid peroxide formation do not inhibit aminopyrine oxidation. Microsomes prepared from sucrose–EDTA homogenates rapidly oxidize aminopyrine, but do not form lipid peroxide when incubated with ascorbate or NADPH. 3. Aminopyrine oxidation is strongly inhibited by p-chloromercuribenzoate, less by iodoacetamide and weakly by N-ethylmaleimide. The site of action of these compounds is considered to be a ferredoxin-type protein. GSH and cysteine also inhibit. 4. Other drugs oxidized by microsomes such as caffeine, phenobarbitone and hexobarbitone had either no or little effect on lipid peroxide formation, but codeine inhibited. 5. Most aliphatic hydrocarbons, alcohols, ketones and aldehydes did not affect lipid peroxide formation, but chloroform and carbon tetrachloride inhibited. 6. Many aromatic compounds inhibited lipid peroxide formation. Only aromatic acids were without any effect and phenols and amines were very strong inhibitors. 7. Induction of lipid peroxide formation in microsomes by incubation with ascorbate or NADPH or by treatment with ionizing radiation leads to a sharp decline in the ability of microsomes to oxidize aminopyrine or hydroxylate aniline. 8. It is considered that the two processes of hydroxylation and lipid peroxide formation are closely linked in microsomes. They probably depend on the same electron-transport chain, and peroxide formation, which involves membrane disintegration, may be part of the normal membrane remodelling process.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRODIE B. B., GILLETTE J. R., LA DU B. N. Enzymatic metabolism of drugs and other foreign compounds. Annu Rev Biochem. 1958;27(3):427–454. doi: 10.1146/annurev.bi.27.070158.002235. [DOI] [PubMed] [Google Scholar]
- Christophersen B. O. The inhibitory effect of reduced glutathione on the lipid peroxidation of the microsomal fraction and mitochondria. Biochem J. 1968 Jan;106(2):515–522. doi: 10.1042/bj1060515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernster L., Orrenius S. Substrate-induced synthesis of the hydroxylating enzyme system of liver microsomes. Fed Proc. 1965 Sep-Oct;24(5):1190–1199. [PubMed] [Google Scholar]
- May H. E., McCay P. B. Reduced triphosphopyridine nucleotide oxidase-catalyzed alterations of membrane phospholipids. I. Nature of the lipid alterations. J Biol Chem. 1968 May 10;243(9):2288–2295. [PubMed] [Google Scholar]
- NASH T. The colorimetric estimation of formaldehyde by means of the Hantzsch reaction. Biochem J. 1953 Oct;55(3):416–421. doi: 10.1042/bj0550416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omura T., Sato R., Cooper D. Y., Rosenthal O., Estabrook R. W. Function of cytochrome P-450 of microsomes. Fed Proc. 1965 Sep-Oct;24(5):1181–1189. [PubMed] [Google Scholar]
- Orrenius S., Dallner G., Ernster L. Inhibition of the TPNH-linked lipid peroxidation of liver microsomes by drugs undergoing oxidative demethylation. Biochem Biophys Res Commun. 1964;14:329–334. doi: 10.1016/s0006-291x(64)80005-x. [DOI] [PubMed] [Google Scholar]
- Siekevitz P. Electron transport systems in microsomes. Origin and functional nature of microsomes. Fed Proc. 1965 Sep-Oct;24(5):1153–1155. [PubMed] [Google Scholar]
- Wills E. D. Lipid peroxide formation in microsomes. General considerations. Biochem J. 1969 Jun;113(2):315–324. doi: 10.1042/bj1130315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills E. D. Lipid peroxide formation in microsomes. The role of non-haem iron. Biochem J. 1969 Jun;113(2):325–332. doi: 10.1042/bj1130325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills E. D. Mechanisms of lipid peroxide formation in animal tissues. Biochem J. 1966 Jun;99(3):667–676. doi: 10.1042/bj0990667. [DOI] [PMC free article] [PubMed] [Google Scholar]


