Abstract
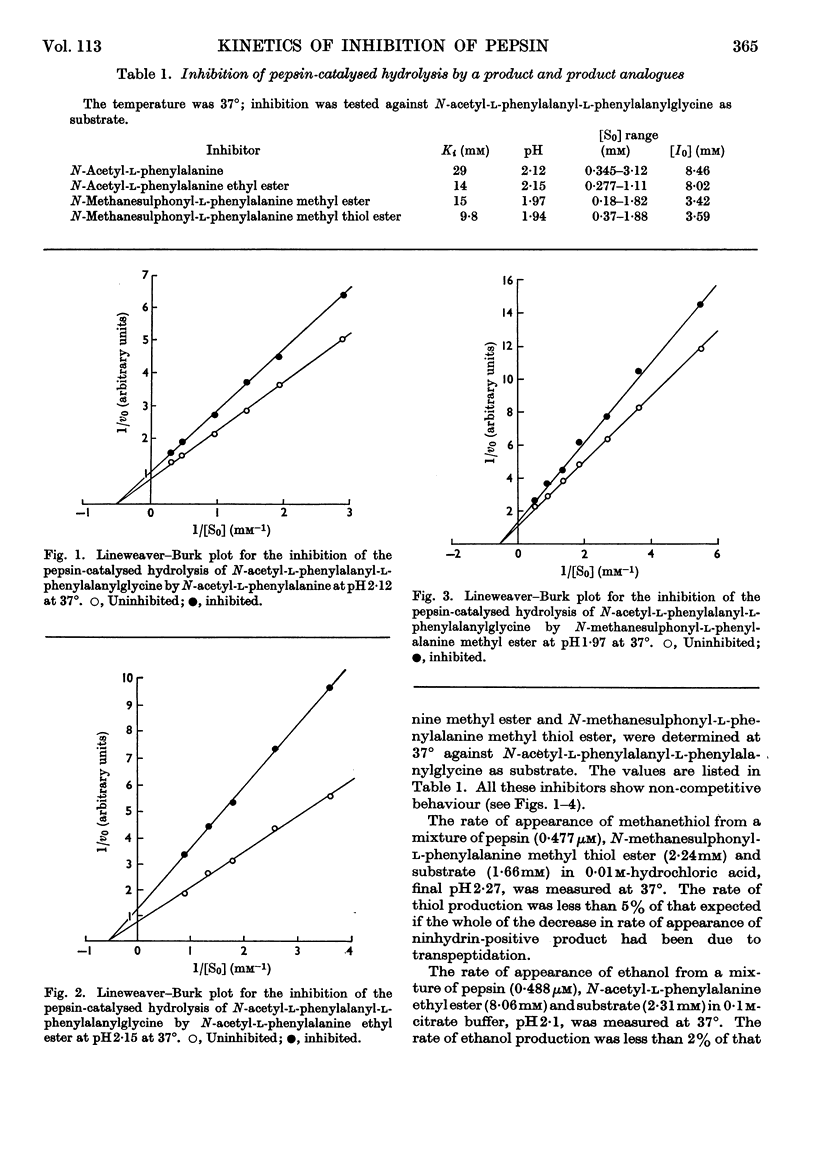
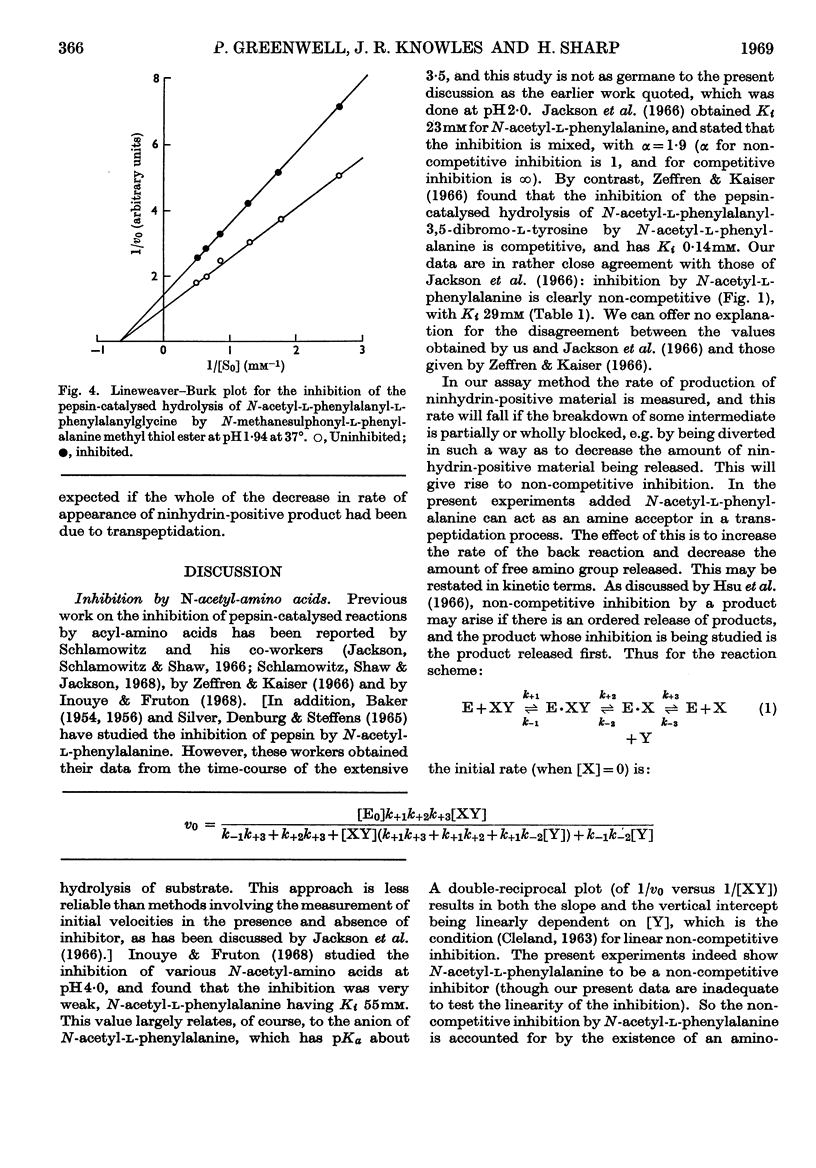
1. The inhibition of pepsin-catalysed hydrolysis of N-acetyl-l-phenylalanyl-l-phenylalanylglycine by products and product analogues was studied. 2. The non-competitive nature of the inhibition by the product N-acetyl-l-phenylalanine confirms an ordered release of products, and points to a common mechanism (involving an amino-enzyme) for pepsin-catalysed transpeptidation and hydrolysis reactions. 3. N-Acetyl-l-phenylalanine ethyl ester is also a non-competitive inhibitor, but here the inhibition is of the `dead-end' type. No ethanol is detectable in reaction mixtures, indicating that this ester cannot act as an amino group acceptor in a transpeptidation process. 4. The same is true for N-methanesulphonyl-l-phenylalanine methyl and methyl thiol esters. No methanethiol is liberated when the methyl thiol ester is present as an inhibitor of the hydrolytic reaction, and the hope that such a thiol ester would effectively trap the amino-enzyme was not fulfilled.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAKER L. E. The kinetics of the action of pepsin on synthetic substrates. J Biol Chem. 1954 Dec;211(2):701–716. [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. II. Inhibition: nomenclature and theory. Biochim Biophys Acta. 1963 Feb 12;67:173–187. doi: 10.1016/0006-3002(63)91815-8. [DOI] [PubMed] [Google Scholar]
- Cornish-Bowden A. J., Greenwell P., Knowles J. R. The rate-determining step in pepsin-catalysed reactions, and evidence against an acyl-enzyme intermediate. Biochem J. 1969 Jun;113(2):369–375. doi: 10.1042/bj1130369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish-Bowden A. J., Knowles J. R. The pH-dependence of pepsin-catalysed reactions. Biochem J. 1969 Jun;113(2):353–362. doi: 10.1042/bj1130353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- FRUTON J. S., FUJII S., KNAPPENBERGER M. H. The mechanism of pepsin action. Proc Natl Acad Sci U S A. 1961 Jun 15;47:759–761. doi: 10.1073/pnas.47.6.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu R. Y., Cleland W. W., Anderson L. Mechanism of action of the nonspecific phosphomonoesterase from potatoes. Biochemistry. 1966 Feb;5(2):799–807. doi: 10.1021/bi00866a055. [DOI] [PubMed] [Google Scholar]
- Inouye K., Fruton J. S. Studies on the specificity of pepsin. Biochemistry. 1967 Jun;6(6):1765–1777. doi: 10.1021/bi00858a027. [DOI] [PubMed] [Google Scholar]
- Inouye K., Fruton J. S. The inhibition of pepsin action. Biochemistry. 1968 May;7(5):1611–1615. doi: 10.1021/bi00845a001. [DOI] [PubMed] [Google Scholar]
- NEUMANN H., LEVIN Y., BERGER A., KATCHALSKI E. Pepsincatalysed transpeptidation of the amino-transfer type. Biochem J. 1959 Sep;73:33–41. doi: 10.1042/bj0730033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILVER M. S., DENBURG J. L., STEFFENS J. J. SPECTROPHOTOMETRIC DETERMINATION OF THE KINETICS OF THE PEPSIN-CATALYZED HYDROLYSIS OF CERTAIN DIPEPTIDE SUBSTRATES. J Am Chem Soc. 1965 Feb 20;87:886–889. doi: 10.1021/ja01082a032. [DOI] [PubMed] [Google Scholar]
- Schlamowitz M., Shaw A., Jackson W. T. The nature of the binding of inhibitors to pepsin and the kinetics of inhibited peptic hydrolysis of N-acetyl-L-phenylalanyl-L-tyrosine. J Biol Chem. 1968 May 25;243(10):2821–2828. [PubMed] [Google Scholar]


