Abstract
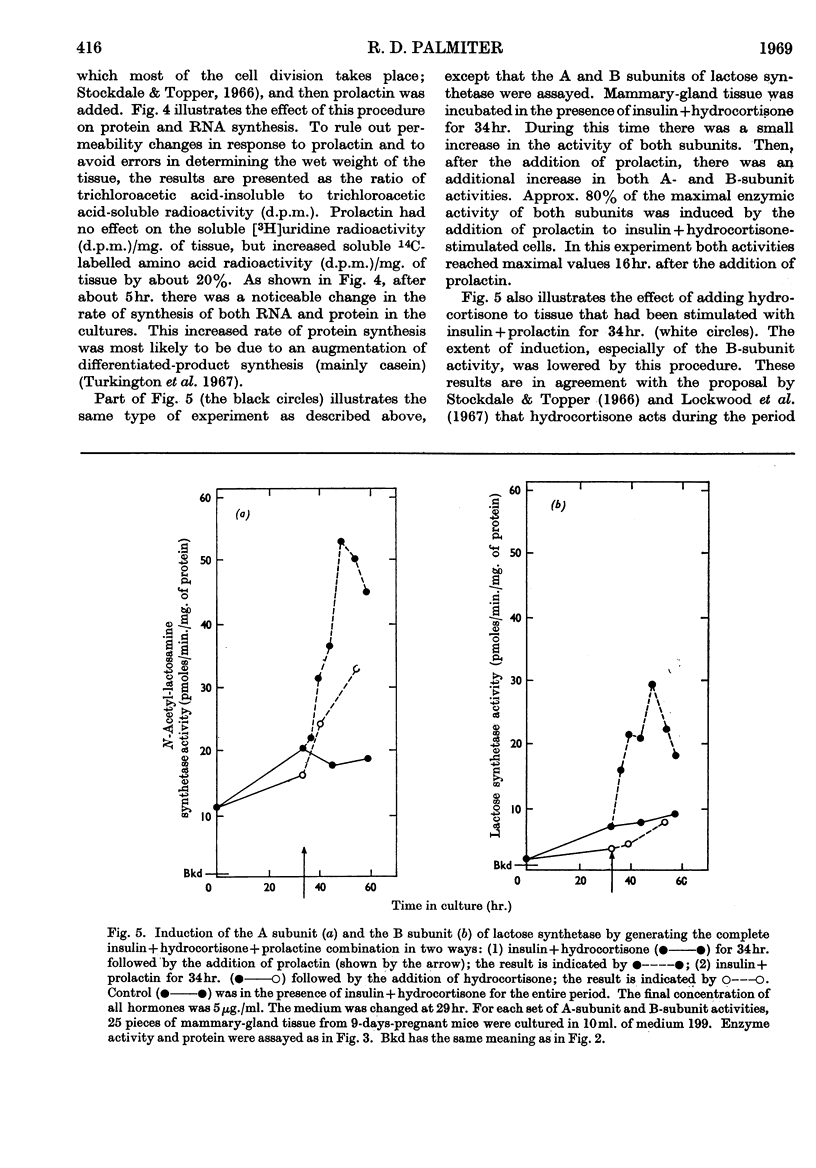
The augmentation of lactose synthetase activity during late pregnancy and lactation was measured by using both a tissue-culture assay and a cell-free assay. The results indicated at least a 100-fold augmentation in specific activity between late pregnancy and lactation. The cell-free assay indicated that the activities of both subunits of this enzyme had increased to 20–30% of the value during lactation by the last day of pregnancy. The tissue-culture assay, however, showed activities only 3–4% of the maximum at the time of parturition. This suggests that not all the enzyme present in the tissue before lactation commenced was active. Since at all stages of pregnancy and lactation the B subunit, α-lactalbumin (which is also a milk protein), was rate-limiting, it is suggested that the rate of lactose synthesis may be linked to the rate of milk-protein synthesis. Both subunits of lactose synthetase could be induced in tissue culture by the hormones insulin+hydrocortisone+prolactin. Of the three hormones, prolactin appeared to be the `trigger' that induced the synthesis of these proteins if the tissue had been stimulated previously by insulin+hydrocortisone.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babad H., Hassid W. Z. Soluble uridine diphosphate D-galactose: D-glucose beta-4-D-galactosyltransferase from bovine milk. J Biol Chem. 1966 Jun 10;241(11):2672–2678. [PubMed] [Google Scholar]
- Bartley J. C., Abraham S., Chaikoff I. L. Biosynthesis of lactose by mammary gland slices from the lactating rat. J Biol Chem. 1966 Mar 10;241(5):1132–1137. [PubMed] [Google Scholar]
- Brew K., Vanaman T. C., Hill R. L. The role of alpha-lactalbumin and the A protein in lactose synthetase: a unique mechanism for the control of a biological reaction. Proc Natl Acad Sci U S A. 1968 Feb;59(2):491–497. doi: 10.1073/pnas.59.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodbeck U., Denton W. L., Tanahashi N., Ebner K. E. The isolation and identification of the B protein of lactose synthetase as alpha-lactalbumin. J Biol Chem. 1967 Apr 10;242(7):1391–1397. [PubMed] [Google Scholar]
- Brodbeck U., Ebner K. E. Resolution of a soluble lactose synthetase into two protein components and solubilization of microsomal lactose synthetase. J Biol Chem. 1966 Feb 10;241(3):762–764. [PubMed] [Google Scholar]
- Brodbeck U., Ebner K. E. The subcellular distribution of the A and B proteins of lactose synthetase in bovine and rat mammary tissue. J Biol Chem. 1966 Dec 10;241(23):5526–5532. [PubMed] [Google Scholar]
- Coffey R. G., Reithel F. J. The lactose synthetase particles of lactating bovine mammary gland. Characteristics of the particles. Biochem J. 1968 Sep;109(2):177–183. doi: 10.1042/bj1090177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey R. G., Reithel F. J. The lactose synthetase particles of lactating bovine mammary gland. Preparation of particles with intact lactose synthetase. Biochem J. 1968 Sep;109(2):169–176. doi: 10.1042/bj1090169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzman R. J. Enzymes of lactose synthesis in the mammary glands of normal and hormonally treated rabbits. Biochem J. 1967 Aug;104(2):24P–25P. [PMC free article] [PubMed] [Google Scholar]
- Juergens W. G., Stockdale F. E., Topper Y. J., Elias J. J. Hormone-dependent differentiation of mammary gland in vitro. Proc Natl Acad Sci U S A. 1965 Aug;54(2):629–634. doi: 10.1073/pnas.54.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn N. J. Lactogenesis in the rat. Metabolism of uridine diphosphate galactose by mammary gland. Biochem J. 1968 Feb;106(3):743–748. doi: 10.1042/bj1060743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosumi K., Kobayashi Y., Baba N. The fine structure of mammary glands of lactating rats, with special reference to the apocrine secretion. Exp Cell Res. 1968 Apr;50(1):177–192. doi: 10.1016/0014-4827(68)90406-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lockwood D. H., Stockdale F. E., Topper Y. J. Hormone-dependent differentiation of mammary gland: sequence of action of hormones in relation to cell cycle. Science. 1967 May 19;156(3777):945–946. doi: 10.1126/science.156.3777.945. [DOI] [PubMed] [Google Scholar]
- Lockwood D. H., Turkington R. W., Topper Y. J. Hormone-dependent development of milk protein synthesis in mammary gland in vitro. Biochim Biophys Acta. 1966 Dec 28;130(2):493–501. doi: 10.1016/0304-4165(66)90245-5. [DOI] [PubMed] [Google Scholar]
- NANDI S., BERN H. A. The hormones responsible for lactogenesis in BALB/cCrgl mice. Gen Comp Endocrinol. 1961 Sep;1:195–210. doi: 10.1016/0016-6480(61)90029-6. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D. Properties of lactose synthetase from mouse mammary gland: role of a proposed third component. Biochim Biophys Acta. 1969 Mar 18;178(1):35–46. doi: 10.1016/0005-2744(69)90129-6. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D. What regulates lactose content in milk? Nature. 1969 Mar 8;221(5184):912–914. doi: 10.1038/221912a0. [DOI] [PubMed] [Google Scholar]
- Sekhri K. K., Pitelka D. R., DeOme K. B. Studies of mouse mammary glands. I. Cytomorphology of the normal mammary gland. J Natl Cancer Inst. 1967 Sep;39(3):459–490. [PubMed] [Google Scholar]
- Stockdale F. E., Topper Y. J. The role of DNA synthesis and mitosis in hormone-dependent differentiation. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1283–1289. doi: 10.1073/pnas.56.4.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TWAROG J. M., LARSON B. L. INDUCED ENZYMATIC CHANGES IN LACTOSE SYNTHESIS AND ASSOCIATED PATHWAYS OF BOVINE MAMMARY CELL CULTURES. Exp Cell Res. 1964 Mar;34:88–99. doi: 10.1016/0014-4827(64)90185-5. [DOI] [PubMed] [Google Scholar]
- Turkington R. W., Brew K., Vanaman T. C., Hill R. L. The hormonal control of lactose synthetase in the developing mouse mammary gland. J Biol Chem. 1968 Jun 25;243(12):3382–3387. [PubMed] [Google Scholar]
- Turkington R. W. Induction of milk protein synthesis by placental lactogen and prolactin in vitro. Endocrinology. 1968 Mar;82(3):575–583. doi: 10.1210/endo-82-3-575. [DOI] [PubMed] [Google Scholar]
- Turkington R. W., Lockwood D. H., Topper Y. J. The induction of milk protein synthesis in post-mitotic mammary epithelial cells exposed to prolactin. Biochim Biophys Acta. 1967 Nov 28;148(2):475–480. doi: 10.1016/0304-4165(67)90144-4. [DOI] [PubMed] [Google Scholar]
- WATKINS W. M., HASSID W. Z. The synthesis of lactose by particulate enzyme preparations from guinea pig and bovine mammary glands. J Biol Chem. 1962 May;237:1432–1440. [PubMed] [Google Scholar]


