Abstract
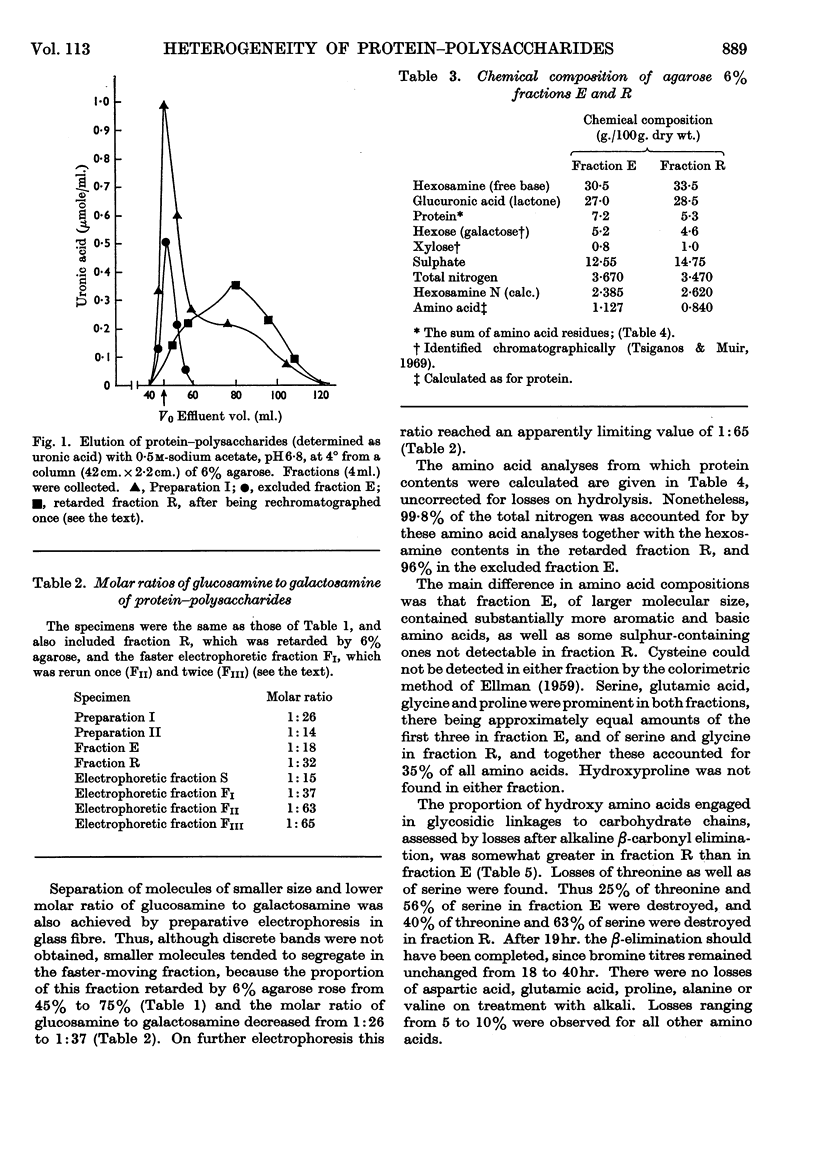
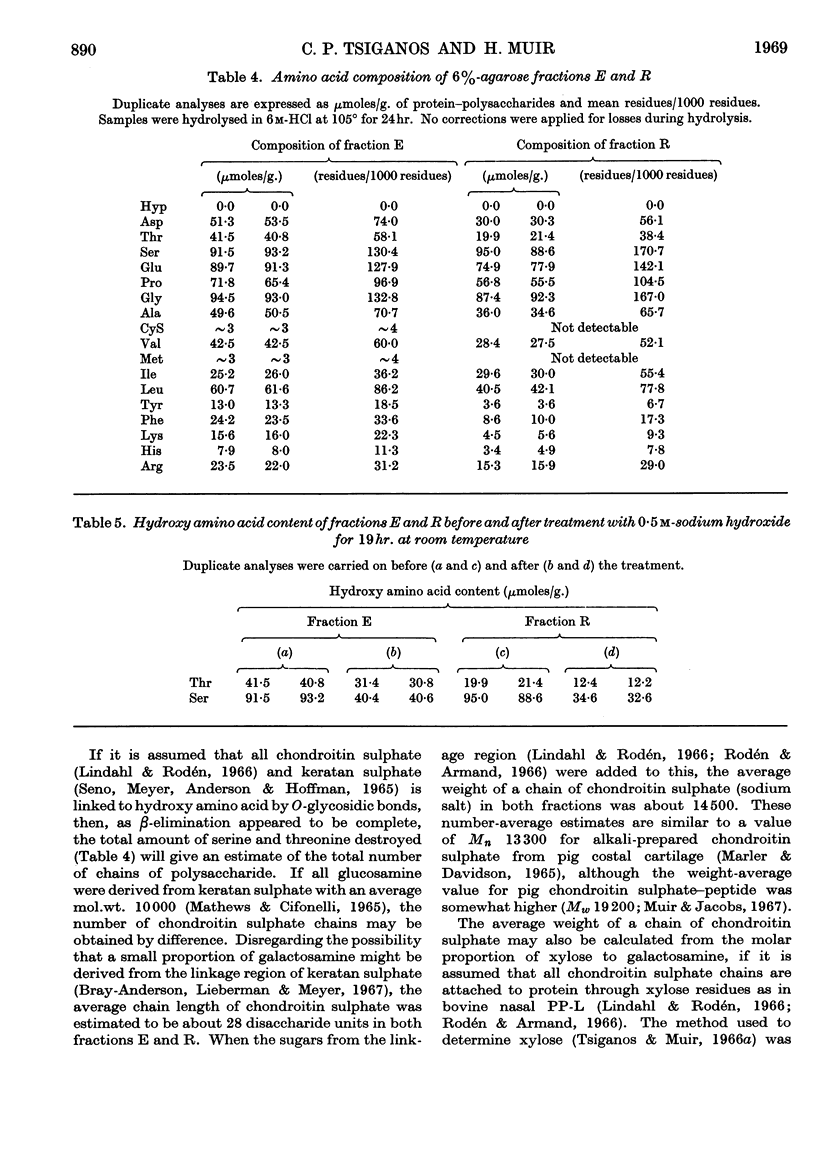
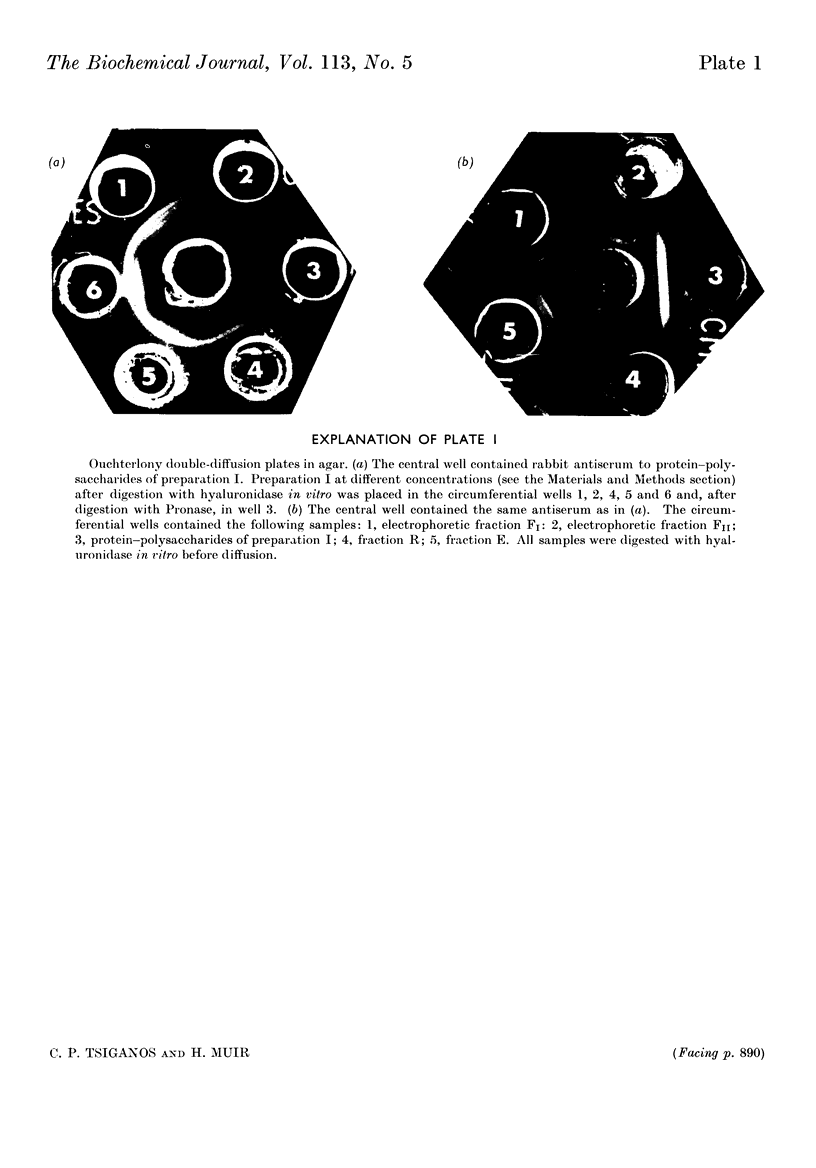
1. Protein–polysaccharides from pig laryngeal cartilage extracted by two procedures described in the preceding paper (Tsiganos & Muir, 1969) were shown to consist of macromolecules of various sizes as assessed by gel filtration in 4% and 6% agarose. 2. A larger proportion of the smaller molecules was present in the preparation obtained by brief extraction in iso-osmotic sodium acetate (procedure I) than in that obtained by more prolonged extraction in 10% (w/v) calcium chloride (procedure II). 3. Two fractions were separated by gel filtration in 6% agarose and by electrophoresis in compressed glass fibre. These fractions differed in chemical composition and in antigenic determinants. The gel-retarded fraction R and that of higher electrophoretic mobility possessed the same single antigen, whereas the gel-excluded fraction E and the slower electrophoretic fraction contained all the antigens of the starting material including that of fraction R. 4. Five N-terminal amino acid residues were identified in preparation I and fraction E, only two of which were present in fraction R. 5. The relative proportions of gel-excluded and gel-retarded fractions did not change when solutions of high ionic strength, urea or guanidine hydrochloride were used for elution. 6. The differences in chemical and amino acid composition between fractions R and E showed that the latter was not a simple aggregate of the former. Fraction E contained more basic and aromatic amino acids, and some methionine and cystine; the last two were absent from fraction R. Hydroxyproline was not detected in either fraction. 7. The number of glycosidic linkages in both fractions was estimated by alkaline β-elimination. Appreciable amounts of threonine as well as serine were destroyed in both fractions. An average chain length for chondroitin sulphate was calculated from the galactosamine content of both fractions and the amounts of hydroxy amino acid destroyed. Average chain lengths were also calculated from the xylose and galactosamine content of each fraction. Each independent method gave a value of approximately 28 disaccharide units for the chain length in both fractions and hence their difference in size could not be explained by differences in the length of carbohydrate chains. 8. All fractions contained glucosamine, which was attributed to keratan sulphate. Content of both protein and keratan sulphate increased with the size of the macromolecules. 9. It is suggested, from these results, that chondroitin sulphate–protein complexes normally exist as a heterogeneous population of macromolecules in cartilage, and that keratan sulphate is involved in the formation of larger molecules.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON B., HOFFMAN P., MEYER K. THE O-SERINE LINKAGE IN PEPTIDES OF CHONDROITIN 4- OR 6-SULFATE. J Biol Chem. 1965 Jan;240:156–167. [PubMed] [Google Scholar]
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Bray B. A., Lieberman R., Meyer K. Structure of human skeletal keratosulfate. The linkage region. J Biol Chem. 1967 Jul 25;242(14):3373–3380. [PubMed] [Google Scholar]
- CALLANAN M. J., CARROLL W. R., MITCHELL E. R. Physical and chemical properties of protamine from the sperm of salmon (Oncorhynchus tschawytscha). J Biol Chem. 1957 Nov;229(1):279–287. [PubMed] [Google Scholar]
- CESSI C., PILIEGO F. The determination of amino sugars in the presence of amino acids and glucose. Biochem J. 1960 Dec;77:508–510. doi: 10.1042/bj0770508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRAENKEL-CONRAT H., HARRIS J. I., LEVY A. L. Recent developments in techniques for terminal and sequence studies in peptides and proteins. Methods Biochem Anal. 1955;2:359–425. doi: 10.1002/9780470110188.ch12. [DOI] [PubMed] [Google Scholar]
- HJERTEN S., MOSBACH R. "Molecular-sieve" chromatography of proteins on colums of cross-linked polyacrylamide. Anal Biochem. 1962 Feb;3:109–118. doi: 10.1016/0003-2697(62)90100-8. [DOI] [PubMed] [Google Scholar]
- HJERTEN S. THE PREPARATION OF AGAROSE SPHERES FOR CHROMATOGRAPHY OF MOLECULES AND PARTICLES. Biochim Biophys Acta. 1964 Mar 30;79:393–398. [PubMed] [Google Scholar]
- HOFFMAN P., MEYER K., LINKER A. Transglycosylation during the mixed digestion of hyaluronic acid and chondroitin sulfate by testicular hyaluronidase. J Biol Chem. 1956 Apr;219(2):653–663. [PubMed] [Google Scholar]
- Heinegård D., Gardell S. Studies on protein-polysaccharide complex (proteoglycan) from human nucleus pulposus. I. Isolation and preliminary characterisation. Biochim Biophys Acta. 1967 Oct 9;148(1):164–171. doi: 10.1016/0304-4165(67)90292-9. [DOI] [PubMed] [Google Scholar]
- Hoffman P., Mashburn T. A., Jr, Meyer K., Bray B. A. Proteinpolysaccharide of bovine cartilage. I. Extraction and electrophoretic studies. J Biol Chem. 1967 Sep 10;242(17):3799–3804. [PubMed] [Google Scholar]
- Hoffman P., Mashburn T. A., Jr, Meyer K. Proteinpolysaccharide of bovine cartilage. II. The relation of keratan sulfate and chondroitin sulfate. J Biol Chem. 1967 Sep 10;242(17):3805–3809. [PubMed] [Google Scholar]
- Lindahl U., Rodén L. The chondroitin 4-sulfate-protein linkage. J Biol Chem. 1966 May 10;241(9):2113–2119. [PubMed] [Google Scholar]
- Loewi G., Muir H. The antigenicity of chondromucoprotein. Immunology. 1965 Aug;9(2):119–127. [PMC free article] [PubMed] [Google Scholar]
- Luscombe M., Phelps C. F. Action of degradative enzymes on the light fraction of bovine septa protein polysaccharide. Biochem J. 1967 Apr;103(1):103–109. doi: 10.1042/bj1030103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATHEWS M. B., LOZAITYTE I. Sodium chondroitin sulfate-protein complexes of cartilage. I. Molecular weight and shape. Arch Biochem Biophys. 1958 Mar;74(1):158–174. doi: 10.1016/0003-9861(58)90210-8. [DOI] [PubMed] [Google Scholar]
- Marler E., Davidson E. A. Structure of a polysaccharide protein complex. Proc Natl Acad Sci U S A. 1965 Aug;54(2):648–656. doi: 10.1073/pnas.54.2.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroudas A., Muir H., Wingham J. The correlation of fixed negative charge with glycosaminoglycan content of human articular cartilage. Biochim Biophys Acta. 1969 May 6;177(3):492–500. doi: 10.1016/0304-4165(69)90311-0. [DOI] [PubMed] [Google Scholar]
- Mathews M. B., Cifonelli J. A. Comparative biochemistry of keratosulfates. J Biol Chem. 1965 Nov;240(11):4140–4145. [PubMed] [Google Scholar]
- Muir H., Jacobs S. Protein-polysaccharides of pig laryngeal cartilage. Biochem J. 1967 May;103(2):367–374. doi: 10.1042/bj1030367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARTRIDGE S. M., DAVIS H. F., ADAIR G. S. The chemistry of connective tissues. 6. The constitution of the chondroitin sulphate-protein complex in cartilage. Biochem J. 1961 Apr;79:15–26. doi: 10.1042/bj0790015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S., Doganges P. T., Schubert M. The separation of new forms of the proteinpolysaccharides of bovine nasal cartilage. J Biol Chem. 1966 Sep 25;241(18):4261–4266. [PubMed] [Google Scholar]
- RUSSELL B., MEAD T. H., POLSON A. A METHOD OF PREPARING AGAROSE. Biochim Biophys Acta. 1964 Apr 4;86:169–174. doi: 10.1016/0304-4165(64)90171-0. [DOI] [PubMed] [Google Scholar]
- Rodén L., Armand G. Structure of the chondroitin 4-sulfate-protein linkage region. Isolation and characterization of the disaccharide 3-O-beta-D-glucuronosyl-D-galactose. J Biol Chem. 1966 Jan 10;241(1):65–70. [PubMed] [Google Scholar]
- Rosenberg L., Schubert M. The proteinpolysaccharides of bovine nucleus pulposus. J Biol Chem. 1967 Oct 25;242(20):4691–4701. [PubMed] [Google Scholar]
- SENO N., MEYER K., ANDERSON B., HOFFMAN P. VARIATIONS IN KERATOSULFATES. J Biol Chem. 1965 Mar;240:1005–1010. [PubMed] [Google Scholar]
- SQUIRE P. G. A RELATIONSHIP BETWEEN THE MOLECULAR WEIGHTS OF MACROMOLECULES AND THEIR ELUTION VOLUMES BASED ON A MODEL FOR SEPHADEX GEL FILTRATION. Arch Biochem Biophys. 1964 Sep;107:471–478. doi: 10.1016/0003-9861(64)90303-0. [DOI] [PubMed] [Google Scholar]
- STEVEN F. S. Conditions for the hydrolysis of DNP-gelatin by Dowex 50 catalysis. Anal Biochem. 1962 Oct;4:316–321. doi: 10.1016/0003-2697(62)90093-3. [DOI] [PubMed] [Google Scholar]
- Serafini-Fracassini A., Peters T. J., Floreani L. The protein-polysaccharide complex of bovine nasal cartilage. Studies on the protein core. Biochem J. 1967 Nov;105(2):569–575. doi: 10.1042/bj1050569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel L. M., Monty K. J. Determination of molecular weights and frictional ratios of proteins in impure systems by use of gel filtration and density gradient centrifugation. Application to crude preparations of sulfite and hydroxylamine reductases. Biochim Biophys Acta. 1966 Feb 7;112(2):346–362. doi: 10.1016/0926-6585(66)90333-5. [DOI] [PubMed] [Google Scholar]
- TREVELYAN W. E., HARRISON J. S. Studies on yeast metabolism. I. Fractionation and microdetermination of cell carbohydrates. Biochem J. 1952 Jan;50(3):298–303. doi: 10.1042/bj0500298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiganos C. P., Muir H. Estimation of pentoses and methylpentoses in biopolymers, in particular of fucose and xylose. Anal Biochem. 1966 Dec;17(3):495–501. doi: 10.1016/0003-2697(66)90184-9. [DOI] [PubMed] [Google Scholar]
- Tsiganos C. P., Muir H. Heterogeneity and the structure of proteoglycans of chondroitin 4-sulphate. Biochem J. 1967 Aug;104(2):15P–16P. [PMC free article] [PubMed] [Google Scholar]
- Tsiganos C. P., Muir H. Studies on protein-polysaccharides from pig laryngeal cartilage. Extraction and purification. Biochem J. 1969 Aug;113(5):879–884. doi: 10.1042/bj1130879. [DOI] [PMC free article] [PubMed] [Google Scholar]



