Abstract
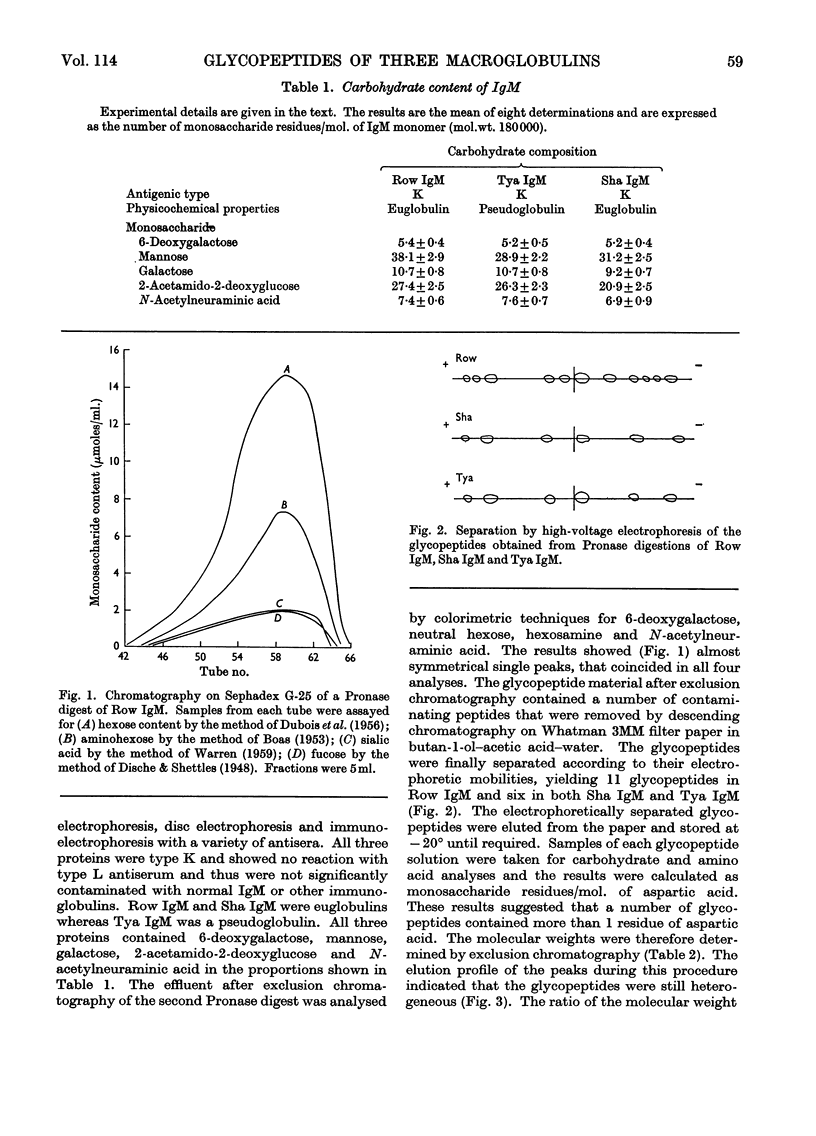
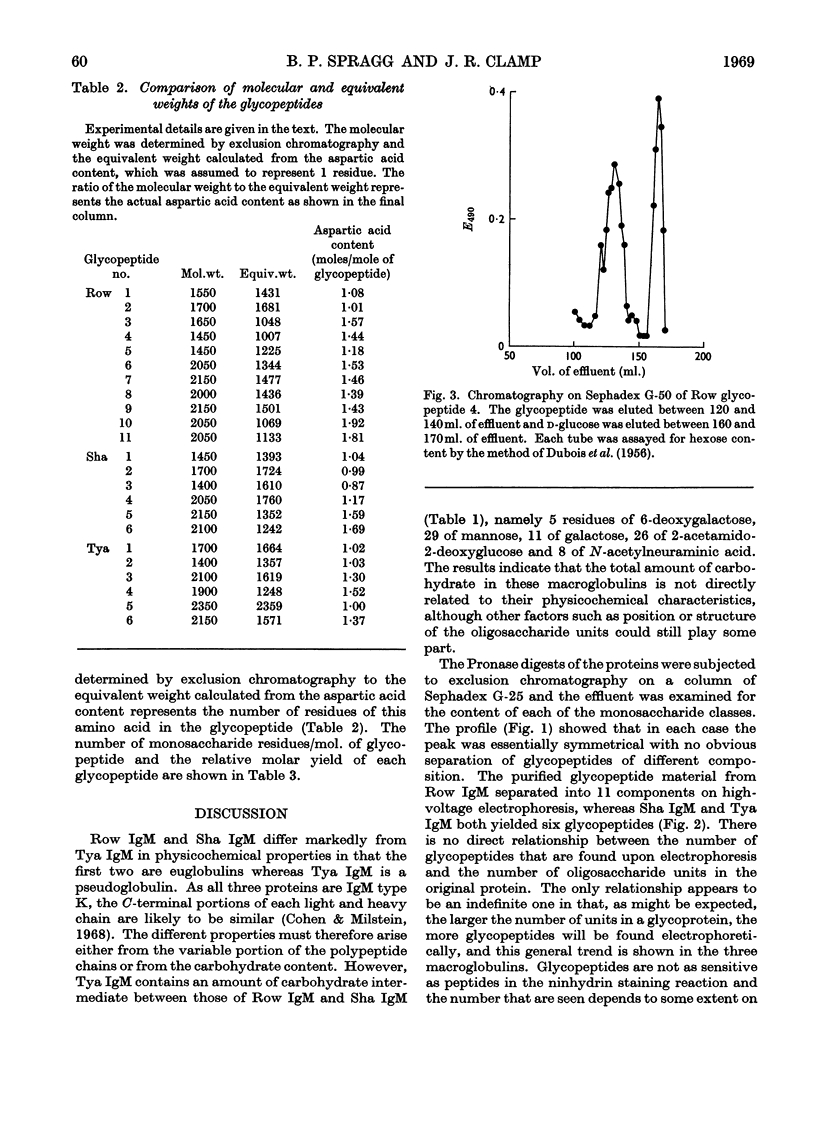
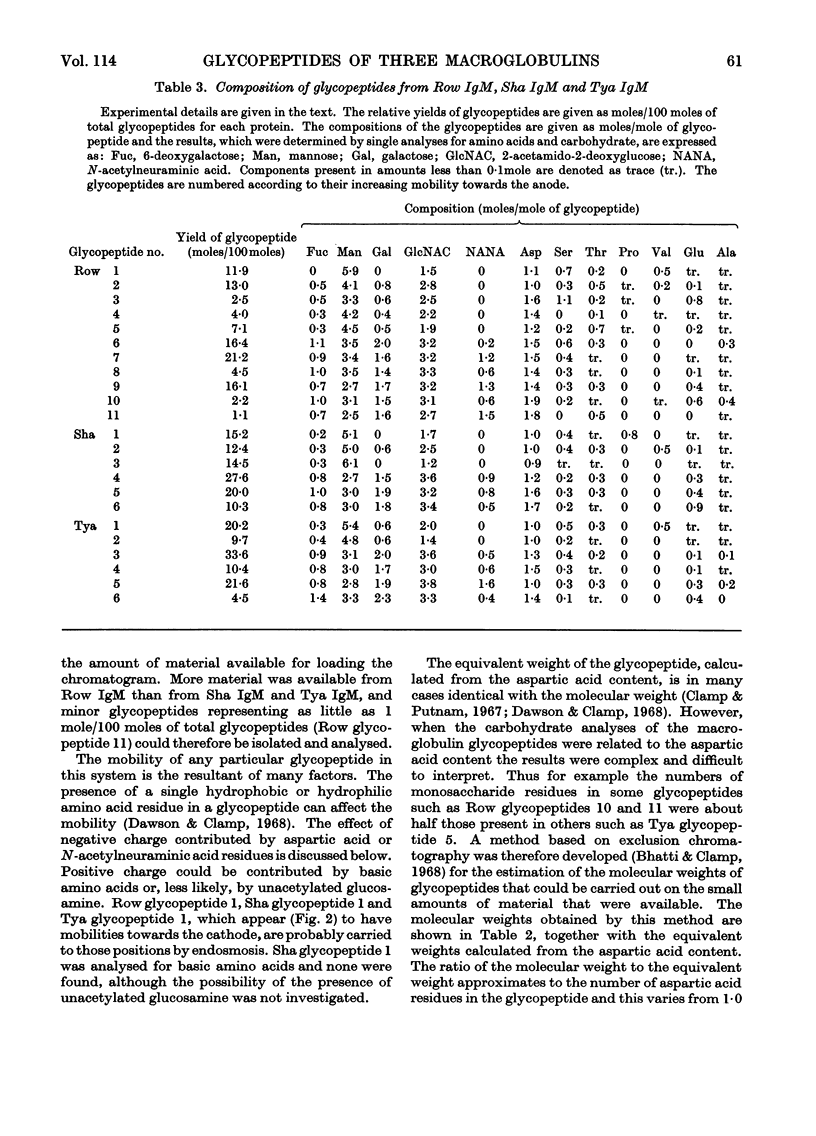
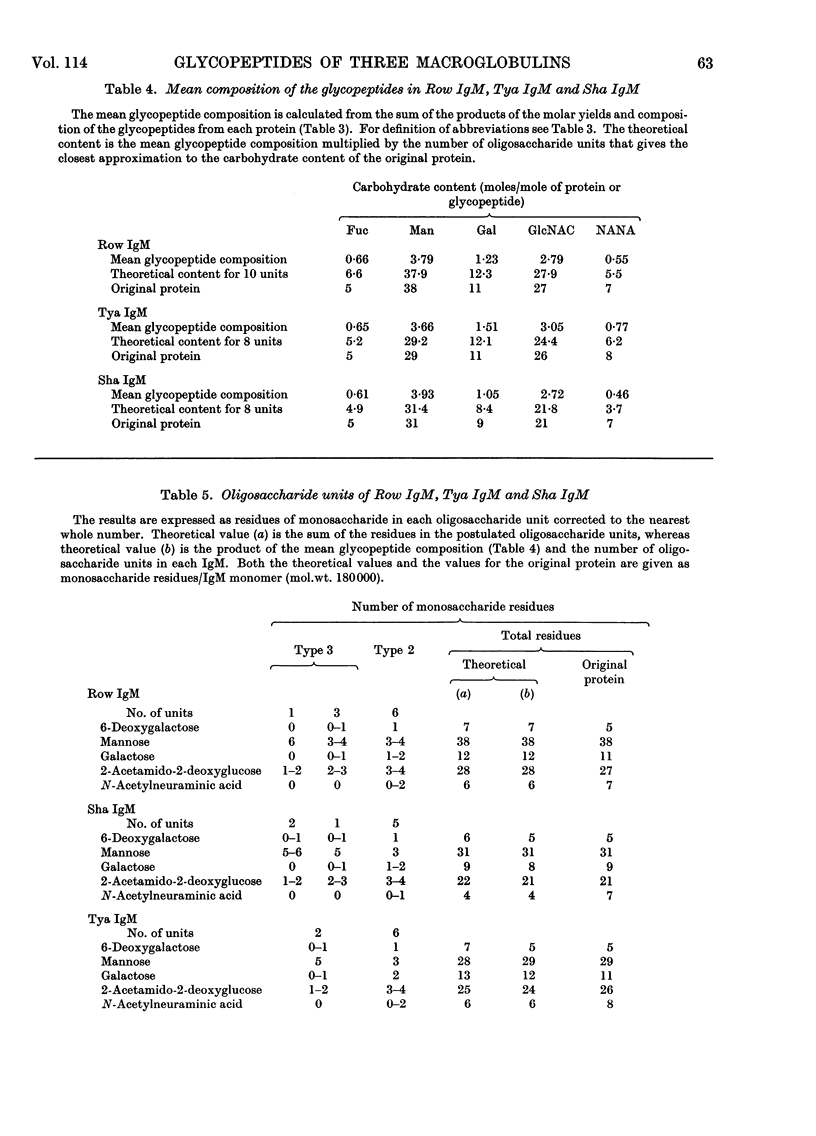
For a monomeric molecular weight of 180000 three type K macroglobulins (IgM) contained 6-deoxygalactose, mannose, galactose, 2-acetamido-2-deoxyglucose and N-acetylneuraminic acid in the molar proportions 5:38:11:27:7 for Row IgM, 5:31:9:21:7 for Sha IgM, and 5:29:11:26:8 for Tya IgM. The first two proteins were euglobulins whereas Tya IgM was a pseudoglobulin, and therefore the total content of carbohydrate does not appear to be related to the physicochemical properties of the proteins. The three proteins appeared to contain different numbers of oligosaccharide units, Row IgM having about ten units/monomer, and Sha IgM and Tya IgM about eight each. All three proteins had two types of oligosaccharide unit, which by analogy with an immunoglobulin A myeloma globulin were called Type 2 and Type 3 respectively. The Type 2 units had molecular weights equal to or greater than 2000 and contained 1 residue of 6-deoxygalactose, 3–4 of mannose, 1–2 of galactose, 3–4 of 2-acetamido-2-deoxyglucose and 0–2 of N-acetylneuraminic acid. The Type 3 units had molecular weights of less than 2000 and contained 0–1 residue of 6-deoxygalactose, 3–6 of mannose, 0–1 of galactose, 1–3 of 2-acetamido-2-deoxyglucose and no N-acetylneuraminic acid. Glycopeptides corresponding to the two types of unit varied in their aspartic acid content in that most of the Type 3 glycopeptides possessed only 1 residue of aspartic acid whereas most of the Type 2 glycopeptides had an average content greater than 1 residue.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOAS N. F. Method for the determination of hexosamines in tissues. J Biol Chem. 1953 Oct;204(2):553–563. [PubMed] [Google Scholar]
- Bhatti T., Clamp J. R. Determinatiion of molecular weight of glycopeptides by exclusion chromatography. Biochim Biophys Acta. 1968 Nov 12;170(1):206–208. doi: 10.1016/0304-4165(68)90176-1. [DOI] [PubMed] [Google Scholar]
- Bourrillon R., Razafimahaleo E. Glycopeptides des immunoglobulines. I. Glycopeptides trypsiques de gamma-M-globuline de Waldenstrom. Bull Soc Chim Biol (Paris) 1967;49(8):1115–1125. [PubMed] [Google Scholar]
- CHAPLIN H., COHEN S., PRESS E. M. PREPARATION AND PROPERTIES OF THE PEPTIDE CHAINS OF NORMAL HUMAN 19 S GAMMA-GLOBULIN (IGM). Biochem J. 1965 Apr;95:256–261. doi: 10.1042/bj0950256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLAMP J. R., PUTNAM F. W. THE CARBOHYDRATE PROSTHETIC GROUP OF HUMAN GAMMA-GLOBULIN. J Biol Chem. 1964 Oct;239:3233–3240. [PubMed] [Google Scholar]
- COHEN S., PORTER R. B. STRUCTURE AND BIOLOGICAL ACTIVITY OF IMMUNOGLOBULINS. Adv Immunol. 1964;27:287–349. doi: 10.1016/s0065-2776(08)60710-5. [DOI] [PubMed] [Google Scholar]
- COHEN S. PROPERTIES OF THE PEPTIDE CHAINS OF NORMAL AND PATHOLOGICAL HUMAN GAMMA GLOBULINS. Biochem J. 1963 Nov;89:334–341. doi: 10.1042/bj0890334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clamp J. R., Dawson G., Hough L. The simultaneous estimation of 6-deoxy-L-galactose (L-fucose), D-mannose, D-galactose, 2-acetamido-2-deoxy-D-glucose (N-acetyl-D-glucosamine) and N-acetylneuraminic acid (sialic acid) in glycopeptides and glycoproteins. Biochim Biophys Acta. 1967 Nov 28;148(2):342–349. doi: 10.1016/0304-4165(67)90129-8. [DOI] [PubMed] [Google Scholar]
- Clamp J. R., Putnam F. W. Glycopeptides of immunoglobulins. Investigations on IgA myeloma globulins. Biochem J. 1967 Apr;103(1):225–229. doi: 10.1042/bj1030225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Milstein C. Structure and biological properties of immunoglobulins. Adv Immunol. 1967;7:1–89. doi: 10.1016/s0065-2776(08)60126-1. [DOI] [PubMed] [Google Scholar]
- DEUTSCH H. F., MORTON J. I. Human serum macroglobulins and dissociation units. I. Physicochemical properties. J Biol Chem. 1958 Apr;231(2):1107–1118. [PubMed] [Google Scholar]
- Dawson G., Clamp J. R. Investigations on the oligosaccharide units of an A myeloma globulin. Biochem J. 1968 Apr;107(3):341–352. doi: 10.1042/bj1070341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson L. A., Morgan W. T. A colorimetric method for the determination of glucosamine and chondrosamine. Biochem J. 1933;27(6):1824–1828. doi: 10.1042/bj0271824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER F., METZGER H. CHARACTERIZATION OF A HUMAN MACROGLOBULIN. I. THE MOLECULAR WEIGHT OF ITS SUBUNIT. J Biol Chem. 1965 Aug;240:3325–3333. [PubMed] [Google Scholar]
- MULLER-EBERHARD H. J., KUNKEL H. G. Ultracentrifugal characteristics and carbohydrate content of macromolecular gamma-globulins. Clin Chim Acta. 1959 Mar;4(2):252–258. doi: 10.1016/0009-8981(59)90138-x. [DOI] [PubMed] [Google Scholar]
- Miller F., Metzger H. Characterization of a human macroglobulin. II. Distribution of the disulfide bonds. J Biol Chem. 1965 Dec;240(12):4740–4745. [PubMed] [Google Scholar]
- Morris J. E., Inman F. P. Isolation of the monomeric subunit of immunoglobulin M with its interchain disulfide bonds intact. Biochemistry. 1968 Aug;7(8):2851–2857. doi: 10.1021/bi00848a022. [DOI] [PubMed] [Google Scholar]
- Smyth D. S., Utsumi S. Structure at the hinge region in rabbit immunoglobulin-G. Nature. 1967 Oct 28;216(5113):332–335. doi: 10.1038/216332a0. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]


