Abstract
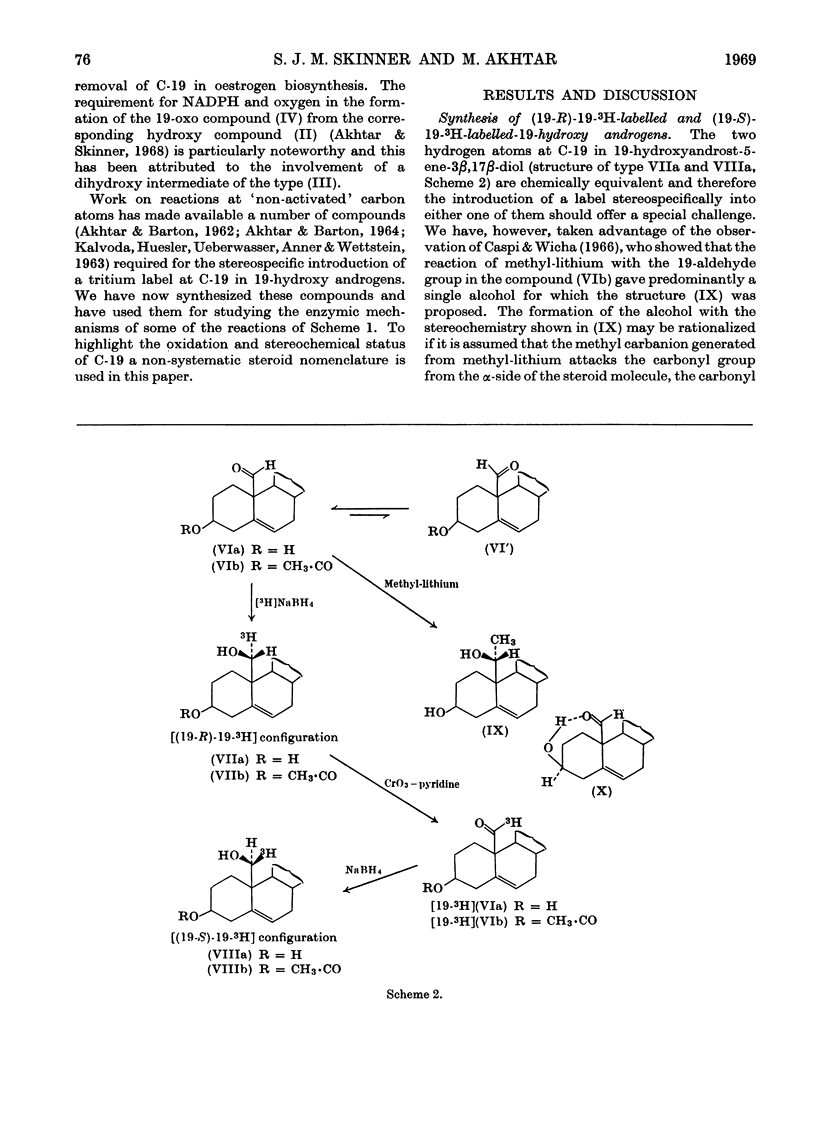
1. The synthesis of a number of 19-substituted androgens is described. 2. A method for the partially stereospecific introduction of a tritium label at C-19 in 19-hydroxyandrost-5-ene-3β,17β-diol was developed. The 19-3H-labelled triol produced by reduction of 19-oxoandrost-5-ene-3β,17β-diol with tritiated sodium borohydride is tentatively formulated as 19-hydroxy[(19-R)-19-3H]androst-5-ene-3β,17β-diol and the 19-3H-labelled triol produced by reduction of 19-oxo[19-3H]-androst-5-ene-3β,17β-diol with sodium borohydride as 19-hydroxy[(19-S)-19-3H]-androst-5-ene-3β,17β-diol. 3. In the conversion of the (19-R)-19-3H-labelled compound into oestrogen by a microsomal preparation from human term placenta more radioactivity was liberated in formic acid (61·6%) than in water (38·4%). In a parallel experiment with the (19-S)-19-3H-labelled compound the order of radioactivity was reversed: formic acid (23·4%), water (76·2%). 4. These observations are interpreted in terms of the removal of the 19-S-hydrogen atom in the conversion of a 19-hydroxy androgen into a 19-oxo androgen during oestrogen biosynthesis. 5. It is suggested that the removal of C-19 in oestrogen biosynthesis occurs compulsorily at the oxidation state of a 19-aldehyde with the liberation of formic acid.
Full text
PDF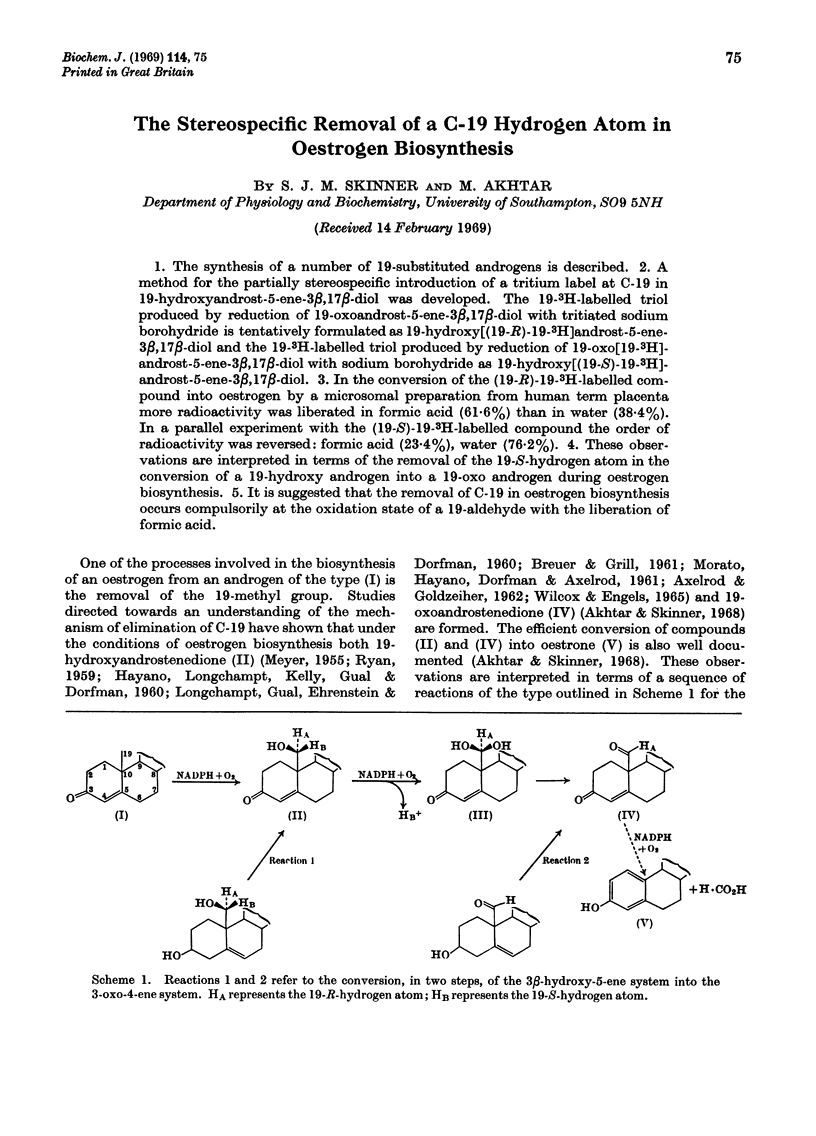




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akhtar M., Skinner S. J. The intermediary role of a 19-oxoandrogen in the biosynthesis of oestrogen. Biochem J. 1968 Sep;109(2):318–321. doi: 10.1042/bj1090318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel O., Ashwell G. Biological mechanisms involved in the formation of deoxysugars. I. Preparation of thymidine diphosphate glucose labeled specifically in carbon 3. J Biol Chem. 1965 Nov;240(11):4123–4127. [PubMed] [Google Scholar]
- LONGCHAMPT J. E., GUAL C., EHRENSTEIN M., DORFMAN R. I. 19-Hydroxy-delta 4-androstene-3,17-dione, an intermediate in estrogen biosynthesis. Endocrinology. 1960 Mar;66:416–419. doi: 10.1210/endo-66-3-416. [DOI] [PubMed] [Google Scholar]
- MEYER A. S. Conversion of 19-hydroxy-delta 4-androstene-3,17-dione to estrone by endocrine tissue. Biochim Biophys Acta. 1955 Jul;17(3):441–442. doi: 10.1016/0006-3002(55)90395-4. [DOI] [PubMed] [Google Scholar]
- MORATO T., HAYANO M., DORFMAN R. I., AXELROD L. R. The intermediate steps in the biosynthesis of estrogens from androgens. Biochem Biophys Res Commun. 1961 Dec 20;6:334–338. doi: 10.1016/0006-291x(61)90140-1. [DOI] [PubMed] [Google Scholar]
- RYAN K. J. Biological aromatization of steroids. J Biol Chem. 1959 Feb;234(2):268–272. [PubMed] [Google Scholar]


