Abstract
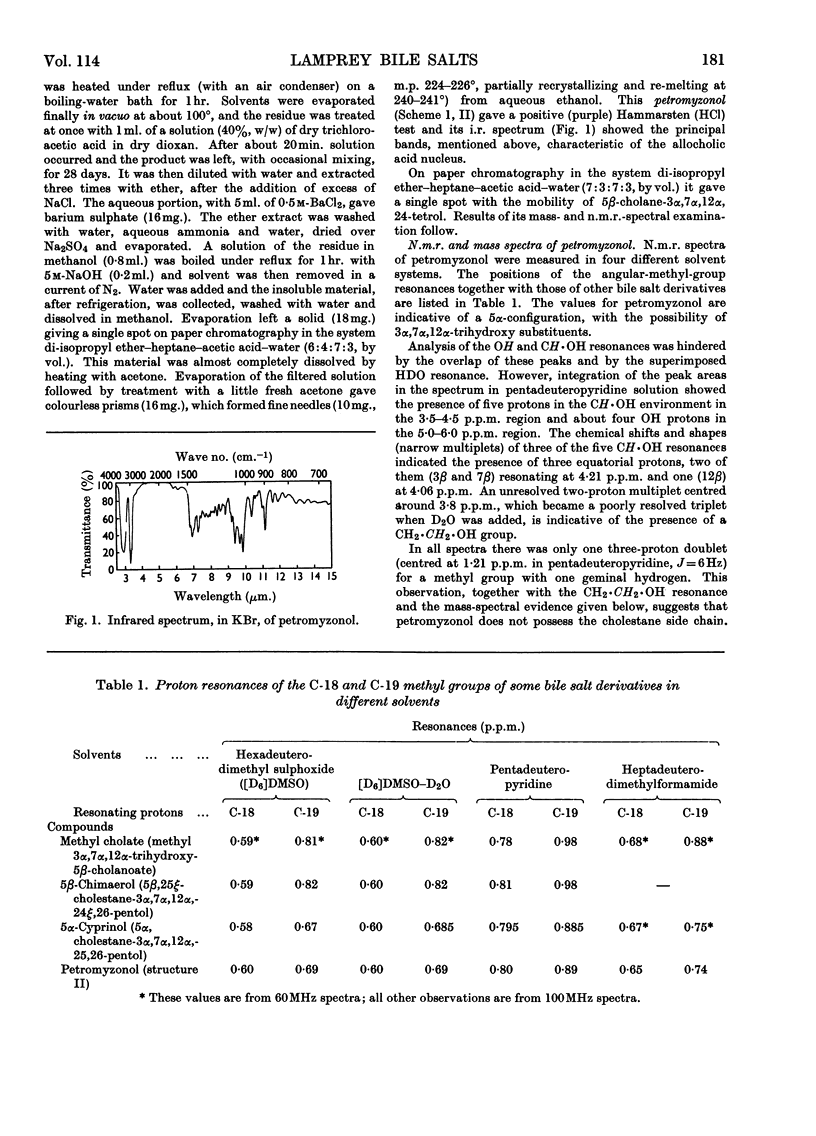
1. Bile salts of Petromyzon marinus L. ammocoetes appeared to consist solely or chiefly of a crystalline substance, whose chromatographic and i.r.-spectral characteristics suggested that it was a monosulphate ester of a bile alcohol having the 3α,7α,12α-trihydroxy pattern of substitution in a 5α-steroid nucleus. 2. This substance on cleavage with dioxan–trichloroacetic acid gave petromyzonol, n.m.r. and mass-spectral examination of which suggested the structure 5α-cholane-3α,7α,12α,24-tetrol. 3. 3α,7α,12α-Trihydroxy-5α-cholanoic acid (allocholic acid) from the lizards Anolis lineatopus lineatopus Gray and Cyclura carinata Harlan (family Iguanidae) was esterified with propan-1-ol and reduced by lithium aluminium hydride to 5α-cholane-3α,7α,12α,24-tetrol, identical with petromyzonol. 4. Chromic acid oxidation of petromyzonol sulphate from lamprey bile, followed by acid hydrolysis, gave 24-hydroxy-5α-cholane-3,7,12-trione; hence the sulphate ester group is at C-24. 5. Petromyzonol sulphate is both primitive and unique: a study of its biogenesis might improve our understanding of evolution at the molecular level.
Full text
PDF
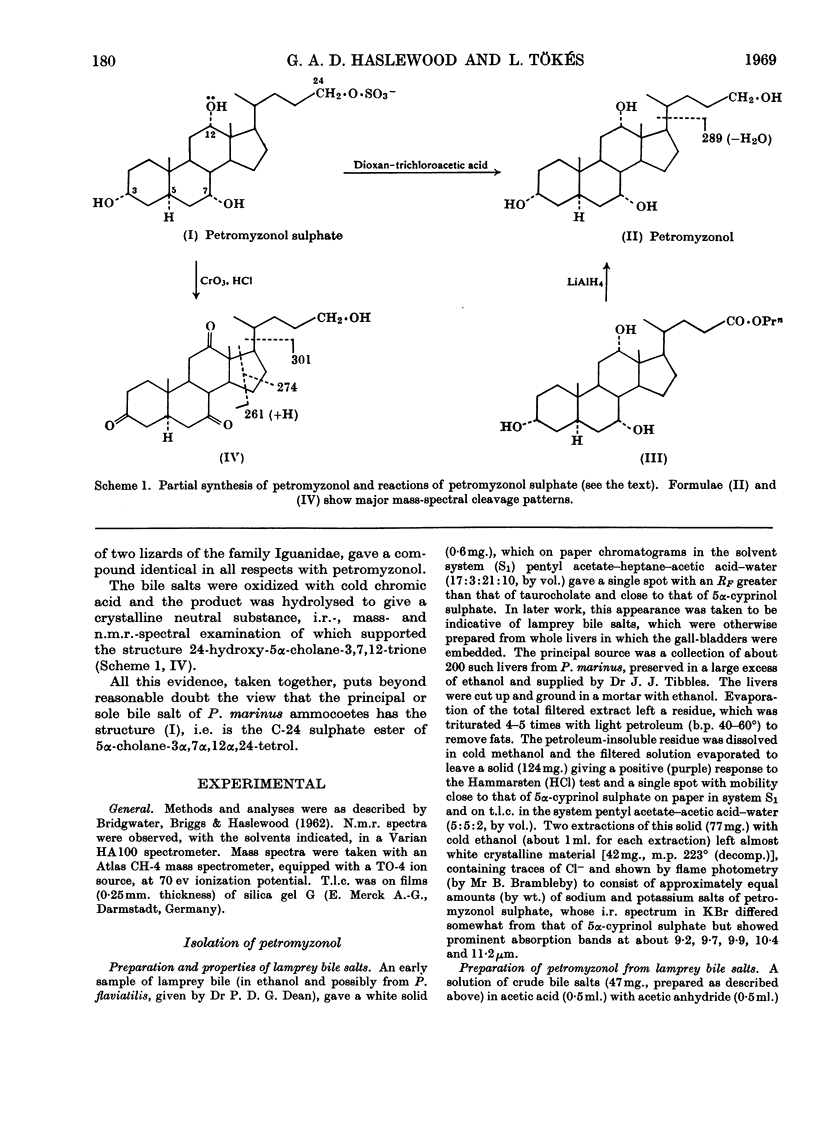



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON I. G., HASLEWOOD G. A. Comparative studiesof 'bile salts'. 15. The natural occurrence and preparation of allocholic acid. Biochem J. 1962 Oct;85:236–242. doi: 10.1042/bj0850236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson I. G., Haslewood G. A. Comparative studies of bile salts. 16-Deoxymyxinol, a second bile alcohol from hagfish. Biochem J. 1969 May;112(5):763–765. doi: 10.1042/bj1120763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson I. G., Haslewood G. A., Cross A. D., Tökés L. New evidence for the structure of myxinol. Biochem J. 1967 Sep;104(3):1061–1063. doi: 10.1042/bj1041061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRIDGWATER R. J., BRIGGS T., HASLEWOOD G. A. Comparative studies of 'bile salts'. 14. Isolation from shark bile and partial synthesis of scymnol. Biochem J. 1962 Feb;82:285–290. doi: 10.1042/bj0820285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djerassi C., Tökés L. Mass spectrometry in structural and stereochemical problems. 93. Further observations on the importance of interatomic distance in the McLafferty rearrangement. Synthesis and fragmentation behavior of deuterium-labeled 12-keto steroids. J Am Chem Soc. 1966 Feb 5;88(3):536–544. doi: 10.1021/ja00955a027. [DOI] [PubMed] [Google Scholar]
- HOSHITA R., KOUCHI M., KAZUNO R. Stero-bile acids and bile sterols. XLIX. Synthesis of 3alpha, 7alpha, 12alpha, 26, 27-pentahydroxycoprostane. J Biochem. 1963 Apr;53:291–294. [PubMed] [Google Scholar]
- Haslewood G. A., Tammar A. R. Comparative studies of bile salts. Bile salts of sturgeons (Acipenseridae) and of the paddlefish Polyodon spathula: a new partial synthesis of 5 beta-cyprinol. Biochem J. 1968 Jun;108(2):263–268. doi: 10.1042/bj1080263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshita T., Shefer S., Mosbach E. H. Conversion of 7-alpha,12-alpha-dihydroxycholest-4-en-3-one to 5-alpha-cholestane-3-alpha, 7-alpha,12-alpha-triol by iguana liver microsomes. J Lipid Res. 1968 Mar;9(2):237–243. [PubMed] [Google Scholar]


